Abstract
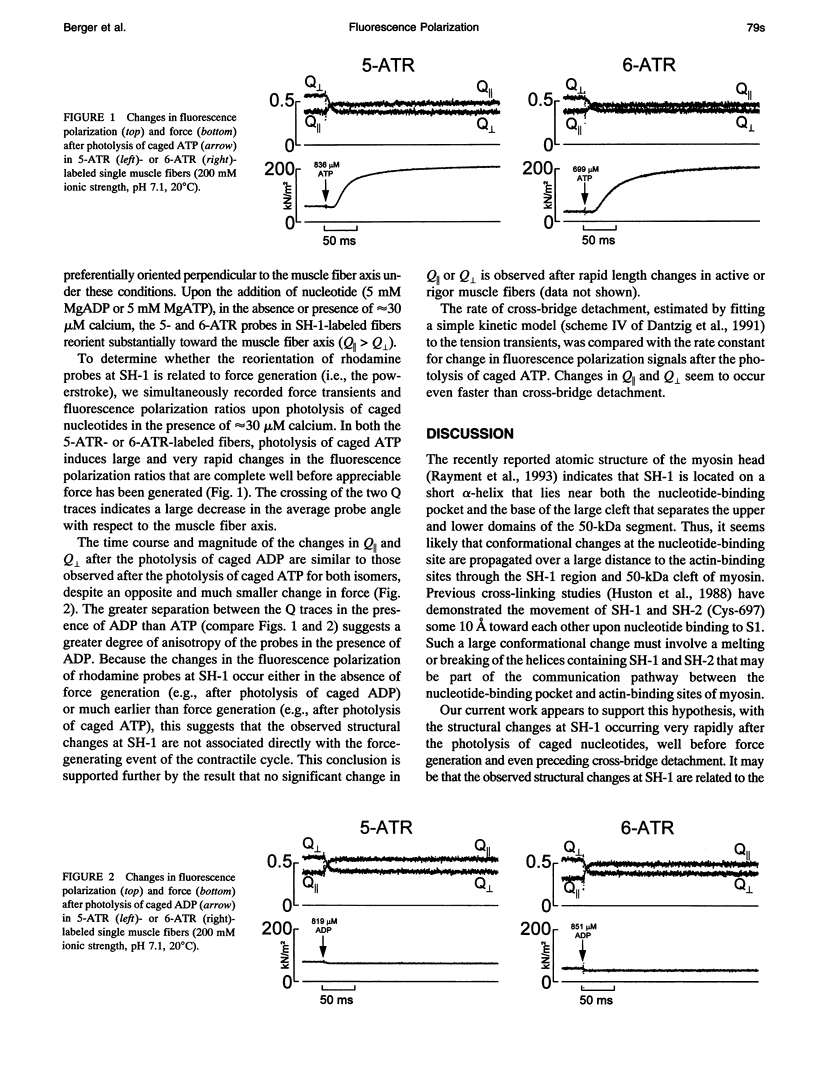
We have used fluorescence polarization to examine orientational changes of the 5- and 6-isomers of acetamidotetramethylrhodamine (ATR) covalently bound to SH-1 (Cys-707 of the myosin heavy chain) in single, skinned fibers from rabbit psoas muscle after rapid length steps or photolysis of caged nucleotides. Similar results were obtained with both the 5- and 6-isomers of ATR. After the photolysis of caged ATP, large and rapid changes in the fluorescence polarization signals were observed and were complete well before appreciable force had been generated. Changes in the fluorescence polarization signals after the photolysis of caged ADP were similar to those after the photolysis of caged ATP, despite an almost negligible change in force. The fluorescence polarization signals remained almost constant after rapid length steps in both rigor and active muscle fibers. These results suggest that structural changes at SH-1 monitored by 5- or 6-ATR are not associated directly with the force-generating event of muscle contraction, but may be involved in the communication pathway between the nucleotide and actin-binding sites of myosin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajtai K., Ilich P. J., Ringler A., Sedarous S. S., Toft D. J., Burghardt T. P. Stereospecific reaction of muscle fiber proteins with the 5' or 6' isomer of (iodoacetamido)tetramethylrhodamine. Biochemistry. 1992 Dec 15;31(49):12431–12440. doi: 10.1021/bi00164a019. [DOI] [PubMed] [Google Scholar]
- Dantzig J. A., Hibberd M. G., Trentham D. R., Goldman Y. E. Cross-bridge kinetics in the presence of MgADP investigated by photolysis of caged ATP in rabbit psoas muscle fibres. J Physiol. 1991 Jan;432:639–680. doi: 10.1113/jphysiol.1991.sp018405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., Trentham D. R. Relaxation of rabbit psoas muscle fibres from rigor by photochemical generation of adenosine-5'-triphosphate. J Physiol. 1984 Sep;354:577–604. doi: 10.1113/jphysiol.1984.sp015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston E. E., Grammer J. C., Yount R. G. Flexibility of the myosin heavy chain: direct evidence that the region containing SH1 and SH2 can move 10 A under the influence of nucleotide binding. Biochemistry. 1988 Dec 13;27(25):8945–8952. doi: 10.1021/bi00425a011. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Tanner J. W., Thomas D. D., Goldman Y. E. Transients in orientation of a fluorescent cross-bridge probe following photolysis of caged nucleotides in skeletal muscle fibres. J Mol Biol. 1992 Jan 5;223(1):185–203. doi: 10.1016/0022-2836(92)90725-y. [DOI] [PubMed] [Google Scholar]
- Thomas D. D. Spectroscopic probes of muscle cross-bridge rotation. Annu Rev Physiol. 1987;49:691–709. doi: 10.1146/annurev.ph.49.030187.003355. [DOI] [PubMed] [Google Scholar]


