Abstract
A phosphatidylinositol 3-phosphate [PI(3)P] 5-kinase gene (CaFAB1) of the most important human pathogenic yeast, Candida albicans, was cloned and sequenced. An open reading frame was detected which encodes a 2,369-amino-acid protein with a calculated molecular mass of 268 kDa and a relative isoelectric point of 6.76. This protein exhibits 38% overall amino acid sequence identity with Saccharomyces cerevisiae Fab1p. We localized the CaFAB1 gene on chromosome R. To determine the influence of the PI(3)P 5-kinase CaFab1p on processes involved in C. albicans morphogenesis and pathogenicity, we sequentially disrupted both copies of the gene. Homozygous deletion of C. albicans CaFAB1 resulted in a mutant strain which exhibited defects in morphogenesis. A Cafab1 null mutant had enlarged vacuoles, an acidification defect, and increased generation times and was unable to form hyphae on different solid media. The sensitivities to hyperosmotic and high-temperature stresses, adherence, and virulence compared to those of wild-type strain SC5314 were not affected.
Candida albicans is the major fungal pathogen in humans, and its medical significance is increasing (34). This dimorphic yeast is capable of causing life-threatening infections in immunocompromised patients, as well as a variety of mucosal infections in healthy individuals. The present evidence suggests that several factors contribute to the virulence of C. albicans; these factors include the ability to switch between different morphogenetic forms, host epithelial and endothelial cell recognition and adhesion, and secretion of proteinases and phospholipases (12, 20, 24, 29, 35). While a number of virulence factors of C. albicans have been characterized, the mechanism which enables this opportunistic fungus to become pathogenic has not been unraveled yet.
In eukaryotic cells many biochemical processes are based on the interactions between cytosolic proteins and intracellular membranes. Diverse cellular processes, such as vesicular transport of proteins, signal transduction, cell adhesion, mitogenesis, growth factor downregulation, cytoskeleton rearrangement, and the osmotic stress response, are processes that demand regulated and reversible docking of cytosolic proteins on specific membranes (13, 15, 44). The proteins bind to cytosolic domains of transmembrane proteins or to the GTP-associated forms of membrane-associated small GTPases. However, it seems that reversible membrane binding of proteins through interactions with specific lipid head groups is an additional mechanism whose importance has increased. In this process phosphatidylinositol (PI) and derivatives of PI that are phosphorylated at positions D-3, D-4, and D-5 play an important role. The different phosphorylated inositols are recognized by different groups of cytosolic proteins, most of which are unknown. However, target proteins of phosphatidylinositol 3-phosphate [PI(3)P] were identified in the yeast Saccharomyces cerevisiae and mammalian cells as proteins that contain a PI(3)P-binding FYVE (Fab1p, YOTB, Vps27p, EEA1) finger domain (9, 42). Two of these proteins, Vac1p and Vps27p, are known to function in Golgi apparatus-to-endosome and endosome-to-vacuole transport processes (38, 45). Another effector protein, Fab1p, functions downstream of ScVps34p as a PI(3)P 5-kinase which regulates vacuolar membrane turnover through the production of phosphatidylinositol 3,5-diphosphate [PI(3,5)P2] (19). Additionally, Fab1p has been shown to be involved in vesicle-mediated sorting of vacuolar hydrolases in endosome-to-vacuole transport via the MVB (multivesicular body) pathway. It was supposed that PI(3,5)P2 recruits or activates proteins which are responsible for the invagination of vesicles on the surface of the endosomes (MVB). It has been proposed that Fab1p may play a role in the specific selection of proteins for transport from the endosomes to the vacuoles (36). Fab1p, therefore, may have a dual role in regulation of both the endosome-sorting pathway and the recycling of the vacuole membrane. Fab1 mutant cells of S. cerevisiae are enlarged and also contain abnormally large vacuoles. In S. cerevisiae fab1 cells the transport of carboxypeptidase Y is affected and growth does not occur at 37°C. The mutated cells are binucleate or aploid (FAB [formation of anucleate and binucleate] cells) (11, 46). Moreover, hyperosmotic stress provokes a rapid burst of PI(3,5)P2 synthesis in S. cerevisiae (14). In accordance with this, vps34 mutants of S. cerevisiae and C. albicans show high osmosensitivity (6, 23).
Recently, we characterized a C. albicans null mutant with a mutant phosphatidylinositol 3-kinase Vps34p which was unable to form hyphae on different solid media while it showed a significantly delayed yeast-to-hypha transition in liquid media. In addition, this mutant was hypersensitive to high-temperature and hyperosmotic stresses and had a strongly decreased ability to adhere to HeLa cells compared to the ability of the wild-type strain SC5314. Moreover, the growth of the null mutant was reduced compared to the growth of the wild-type strain. Finally, we presented evidence that CaVPS34 of C. albicans is essential for pathogenicity of the yeast as a Cavps34 null mutant was shown to be avirulent in a mouse model of systemic infection (6, 7, 17). Therefore, it seemed interesting to investigate the role of the PI(3)P 5-kinase Fab1p in the virulence and pathogenicity of C. albicans.
In this paper, we describe the isolation of a PI(3)P 5-kinase from C. albicans homologous to Fab1p from S. cerevisiae and the construction of a Cafab1 null mutant. In addition, we elucidate cellular functions of C. albicans Fab1p, particularly with regard to its potential influence on virulence determinants.
MATERIALS AND METHODS
Strains and growth conditions.
C. albicans strains SC5314 and CAI-4 (18) used in this study were grown either in YPD medium (2% [wt/vol] dextrose, 2% [wt/vol] peptone, 1% [wt/vol] yeast extract), in SD medium (0.7% [wt/vol] yeast nitrogen base without amino acids [Difco Laboratories, Detroit, Mich.], 2% [wt/vol] glucose, 1 M sorbitol), in YNB (SD medium without 1 M sorbitol), or in Sabouraud dextrose broth (Difco) at 28°C. SD medium, YNB, and Sabouraud dextrose medium were supplemented with 20 μg of uridine per ml for Ura− strains. Selection of uridine auxotrophs was carried out on YNB plates containing uridine and 1 mg of 5-fluoroorotic acid (Sigma, Deisenhofen, Germany) per ml. Growth was monitored by counting cells with a hemocytometer. Hyphal growth was induced by diluting late-exponential-phase cultures grown at 28°C 10-fold either into fresh YPD or Sabouraud dextrose medium supplemented with 10 to 15% (wt/vol) fetal calf serum (FCS) or into Spider medium (1% [wt/vol] nutrient broth, 0.2% [wt/vol] K2HPO4, 1% [wt/vol] mannitol) at 37°C. To induce hyphal growth on solid medium, cells were grown overnight in YPD medium at 28°C, washed, diluted, and spread either on Spider medium plates (1% [wt/vol] nutrient broth, 0.2% [wt/vol] K2HPO4, 1.35% [wt/vol] agar, 1% [wt/vol] mannitol) or on YPD medium plates supplemented with 10% (wt/vol) FCS. Between 20 and 100 cells per plate were incubated at 37°C for at least 7 days. The sensitivities of the mutants to ions were tested on YPD medium plates. S. cerevisiae strains were grown on YNB plates (2% [wt/vol] agar) supplemented with essential amino acids or the nucleotide base required by each strain. Escherichia coli XL1-Blue {supE44 hsdR17 recA1 endA1 gyrA46 thi relA1 lac F′ [proAB+ lacIq M15 Tn10(Tetr)]} (Stratagene, La Jolla, Calif.) was used for cloning and C. albicans strain 1161 was used to prepare a library in a fosmid vector (constructed by M. Strathmann, Stanford University, Palo Alto, Calif.; internet address of fosmid library screening database, http://alces.med.umn.edu/Candida.html).
Cloning and sequencing of CaFAB1.
A database with partial DNA sequences from C. albicans (http://alces.med.umn.edu//bin/gbrowse) contains a sequence with homology to the FAB1 gene of S. cerevisiae. This sequence was used to construct two primers for PCR. PCR amplification was carried out to isolate an FAB1 probe from C. albicans SC5314. The PCR mixtures included 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 50 mM KCl, each deoxynucleoside triphosphate at a concentration of 0.2 mM, each primer at a concentration of 0.2 M, and 1 U of Taq polymerase. Thirty cycles were performed, and each cycle consisted of 30 s of denaturation at 95°C, 1 min of annealing at 42°C, and 2 min of elongation at 72°C. A 369-bp product (nucleic acid positions 3079 to 3447) was amplified with the following oligonucleotides: FAB2 (5′-GAAGATGCCGGTATCACTGTG-3′) and FAB1 (5′-GGGATTCTC TGACGGCTT-3′). The product was cloned into plasmid pCRScriptDirectSK(+) (Stratagene) and used to screen a C. albicans 1161 fosmid library (constructed by M. Strathmann). An approximately 22.0-kb XbaI probe-reactive fragment and a 6.0-kb EcoRI probe-reactive fragment were subcloned, yielding plasmids pFX1 and pFE1. The complete EcoRI fragment and parts of the XbaI fragment were subsequently sequenced by the dideoxy chain termination method (40) by using an automated sequencer (MWG Biotech, Ebersberg, Germany) and a Thermo-Sequenase fluorescently labeled primer cycle sequencing kit (Amersham Pharmacia Biotech, Bucking, England). All other recombinant DNA procedures were carried out by using standard protocols (39). A homology search, a Block search, and alignments were performed by using the GCG software package (Genetics Computer Group Inc., Madison, Wis.), the BLOCK search program (Fred Hutchinson Cancer Research Center, Seattle, Wash.) (22), and the BLAST nucleotide sequence similarity search (1).
Electrophoretic karyotyping.
Separation of chromosomes with a contour-clamped homogeneous electric field apparatus (Bio-Rad, Munich, Germany) was performed by using a running time of 24 h at 150 V and a pulse time of 120 s, followed by 40 h at 150 V with a pulse time of 240 s. The gel was run by using a 120° rotation angle at 10°C.
Construction of plasmids pFD1 and pFD2.
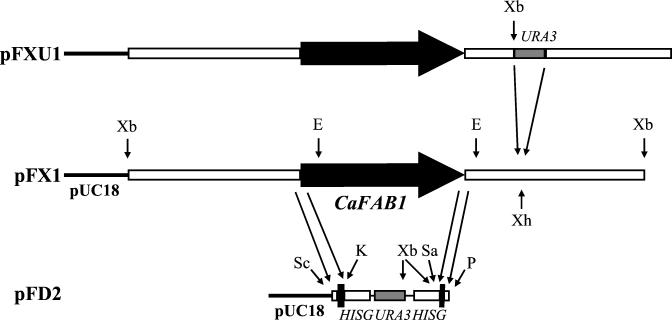
We amplified the 3′ region of CaFAB1 by PCR using chromosomal DNA of C. albicans SC5314 as a template and primers 1 and 2. The primer 1 sequence was 5′-AAAACTGCAGCGGTTGTGTATACTTGATAACACC-3′ (the underlined sequence is complementary to the genomic sequence of the CaFAB1 gene at positions −134 to −111), and the primer 2 sequence was 5′-ACGCGTCGACTTTGATTGGTGGGAATAACGAGG-3′ (positions 6978 to 7001). The resulting 287-bp PCR product was PstI/SalI digested and cloned into the disruption vector pMB-7, yielding pFD1 (18). Primers 3 and 4 were used to amplify the 5′ region. The primer 3 sequence was 5′-GCGAGCTCGCCACAACAGTCCAAGCAGCCCCC-3′ (positions −124 to −101), and the primer 4 sequence was 5′-CGGGGTACCCCTTCATTATGAGTGCTATCCAGT-3′ (positions 272 to 249).This yielded a 413-bp product, which was digested with SacI and KpnI and cloned into plasmid pFD1, resulting in pFD2 (Fig. 1). For gene disruption plasmid pFD2 was cut with SacI and PstI (Fig. 1).
FIG. 1.
Disruption of the FAB1 gene in C. albicans: restriction maps of plasmids pFX1, pDF2, and pFXU1, illustrating the strategy for disruption and reintegration of CaFAB1. For pFX1 the CaFAB1 gene (thick solid arrow, coding region; open boxes, noncoding regions) was cloned into pUC18, yielding plasmid pFX1. For pFD2 the 5′ and 3′ regions of CaFAB1 obtained by PCR were cloned in front of or behind the hisG-URA3-hisG cassette (4.1 kb) in the disruption vector pMB-7. For PFXU1 the URA3 gene (1.4-kb RsaI fragment from pMB-7) was cloned into pFX1 digested with XhoI approximately 2.3 kb downstream of the stop codon of CaFAB1. The labeled arrows indicate the locations of restriction sites, as follows: E, EcoRI; K, KpnI; P, PstI; Sa, SalI; Sc, SacI; Xb, XbaI; Xh, XhoI.
Transformation of C. albicans and selection of Ura− auxotrophs.
The methods used for transformation of C. albicans and selection of Ura− auxotrophs were the methods described previously (3, 16, 43).
Disruption and reintegration of CaFAB1.
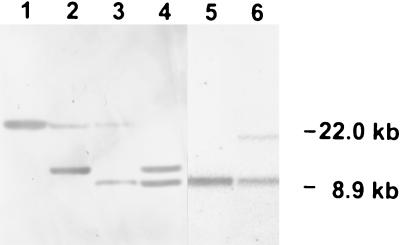
To disrupt CaFAB1, the previously described hisG-URA3-hisG cassette was used in a multistep procedure (18). A 4.7-kb SacI/PstI fragment of pDF2 containing the URA blaster flanked by short sequences from the 5′ and 3′ ends of CaFAB1 and portions of the promoter and terminator was used to transform C. albicans Ura− strain CAI-4 (Fig. 1). After selection on SD medium containing 1 M sorbitol, the resulting Ura+ transformants were examined for gene replacement by Southern analysis of XbaI-digested chromosomal DNA performed with the 0.4-kb PCR product obtained with primers 3 and 4 as a probe (Fig. 2). Southern hybridization was also used to evaluate segregants and transformants of the later disruption steps. In the first step one allele of CaFAB1 was replaced by the hisG-URA3-hisG cassette (strain CAF1). Strain CAF1 was plated on 5-fluoroorotic acid-containing medium for isolation of Ura− segregants (strain CAF2). A second transformation with the same disruption construct led to isolation of a Cafab1 null mutant (strain CAF3). Again, Ura− segregants were selected (strain CAF4).
FIG. 2.
Southern analysis of XbaI-digested chromosomal DNA from the following C. albicans strains: parental strain CAI-4 (lane 1), CAF1 FAB1/fab1::hisG-URA3-hisG (lane 2), CAF2 FAB1/fab1::hisG (lane 3 ), CAF3 fab1::hisG-URA3-hisG/fab1::hisG (lane 4), CAF4 fab1::hisG/fab1::hisG (lane 5), and CAF5 fab1::hisG/FAB1-URA3 (lane 6). The blot was hybridized with the [α-32P]dCTP-labeled XbaI insert of plasmid pFX1.
A 1.4-kb fragment containing the CaURA3 gene was isolated after digestion of pMB-7 with RsaI. The fragment was cloned into pFX1 digested with XhoI (approximately 2.3 kb downstream of the stop codon of CaFAB1) and filled with the Klenow fragment, yielding plasmid pFXU1. This plasmid was digested with KpnI and SphI and transformed into CAF4. Six reintegrants were obtained (strain CAF5).
Adherence assay.
The adherence fluorescence assay was carried out essentially as previously described (4). Briefly, C. albicans cells from an overnight culture in YPD medium at 28°C were washed and diluted into fresh medium containing 10% (wt/vol) FCS. A suspension containing 2 × 106 cells/ml was then preincubated for 1 h at 37°C. Micro test plates containing 1 × 104 HeLa cells per well were washed once with 1× PBS buffer (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4; pH 7.4) and then filled with 200 μl of the culture suspension. After incubation at 37°C for 4 h, the Candida cells were stained by adding 25 μg of Calcofluor white (Sigma) per ml and incubated for an additional 30 min. Nonadherent Candida cells were then removed by washing the plates twice with 1× PBS. Finally, the amount of adherent fluorescent cells was determined by using an automatic fluorescence reader (FluoroScan Labsystems, Helsinki, Finland) with a filter set consisting of a 360-nm excitation filter and a 460-nm emission filter. For comparison, the adherence of wild-type strain SC5314 was defined as 100%. The significance of the observed differences between the strains tested was determined by the Student t test. A P value of ≤0.025 was considered significant.
Virulence studies.
Male outbred NMRI mice (Harlan-Winkelmann, Borchen, Germany) that were 6 weeks old were housed at a density of five mice per cage and were checked daily. Strains of C. albicans were grown in Sabouraud dextrose broth at 28°C until the late logarithmic phase. Cells were washed three times and resuspended in 0.9% NaCl, and 200-μl portions of suspensions containing 5 × 106, 5 × 105, and 5 × 104 cells were used to infect immunocompetent mice by intravenous injection into the lateral tail vein. Survival was monitored for 21 days. For comparison of survival curves the log rank test was used (37). A P value of ≤0.05 was considered significant. To quantify kidney colonization by C. albicans, mice were sacrificed 72 h after injection, and the organs were homogenized in 3 ml of physiological NaCl buffer. Serially diluted suspensions were then plated on YPD agar. After 3 days of growth at 28°C the numbers of Candida colonies were counted.
Fluorescent labeling and microscopy.
Growing yeast cells from an overnight culture in YPD medium (30°C) and cells after induction of hyphal growth in YPD medium supplemented with 15% FCS (37°C) were harvested by 5 min of centrifugation at 3,800 × g and washed twice in physiological NaCl. Then cells (106 cells/ml) were resuspended in 10 mM HEPES buffer (pH 7.4) containing 5% glucose. 4′,6-Diamidino-2-phenylindole (DAPI) (Molecular Probes, Eugene, Oreg.) was used to stain nuclei by a method adapted from a method described previously (30). The samples were mounted on a glass slide, treated for 30 min at 60°C, fixed with an ethanol-acetic acid solution (3:1, vol/vol) for 15 min, and washed in McIlvaine buffer (pH 7.0). Staining was performed directly on the glass slide by adding 0.1 ml of McIlvaine buffer (pH 7.0) containing 0.5 μg of DAPI per ml for 15 min at 37°C. Samples were observed for fluorescence with a fluorescence microscope (Axioplan 1; Zeiss, Jena, Germany) equipped with a BP365 excitation filter, an FT395 beam splitter, and an LP397 emission filter.
For staining with Quinacrine, 500 μl of yeast growing cells from YPD medium overnight cultures and 500 μl of hyphal growing cells were harvested by centrifugation for 5 min at 3,800 × g and resuspended in 500 μl of YPD medium (pH 7.6). Then 2.5 μl of a Quinacrine solution (20 mg/ml; Molecular Probes) was added. After incubation for 5 min at room temperature and centrifugation (3,800 × g, 5 min), the pellet was resuspended in YPD medium. Images were collected with a fluorescence microscope (Axioplan 1; Zeiss) equipped with a BP450-490 excitation filter, an FT510 beam splitter, and a BP515-565 emission filter.
Nucleotide sequence accession number.
The nucleotide sequence data for the CaFAB1 gene have been deposited in the EMBL database under accession no. AJ320541.
RESULTS
Cloning and sequencing of a PI(3)P 5-kinase homologue in C. albicans.
An ScFAB1 homologous sequence from a database with short DNA sequences from C. albicans was used to construct primers FAB1 and FAB2. PCR amplification was carried out to isolate a CaFAB1 probe from C. albicans SC5314. To obtain the complete open reading frame of the CaFAB1 gene, the cloned sequence was used as a probe to screen a C. albicans 1161 fosmid library. Three clones (8B2, 10F7, and 20H2) were isolated and analyzed by Southern hybridization. Evaluation of the data for the fosmid library showed that only the genes from fosmid 8B2 are localized on chromosome R. To confirm the localization of CaFAB1 on chromosome R, we performed Southern hybridization. Chromosomes from C. albicans strain SC5314 were separated by pulsed-field gel electrophoresis. Southern analysis showed that the CaFAB1 probe hybridized with the second chromosome band of C. albicans SC5314, which corresponds to chromosome R (10) (data not shown).
To test whether the fosmids contained a second CaFAB1 gene or a gene highly homologous to CaFAB1, Southern blot analysis of chromosomal DNA of C. albicans strain SC5314 was performed by using the PCR product as a probe and by using the hybridization and washing conditions used for fosmid screening. The resulting hybridization pattern for five samples restricted by five different restriction enzymes indicated that CaFAB1 is present as a single-copy gene in each haploid genome in C. albicans and that no other homologous genes occur in the C. albicans genome.
An approximately 22.0-kb XbaI probe-reactive fragment and a 6.0-kb EcoRI probe-reactive fragment were subcloned, yielding plasmids pFX1 (Fig. 1) and pFE1. When we sequenced the 6,029-bp EcoRI fragment of pFE1 and 1,586 bp from the XbaI fragment of pFX1, we found an entire 7,110-bp open reading frame coding for a predicted protein consisting of 2,369 amino acids with a calculated molecular mass of 268 kDa and a relative isoelectric point of 6.76. One TATA box at nucleotide positions −62 to −58 and two potential CAAT boxes at positions −176 to −179 and −209 to −206 were located. Amino acid sequence comparison revealed 38% overall identity and 47% similarity between the identified open reading frame and the PI(3)P 5-kinase Fab1p from S. cerevisiae (46). Therefore, this open reading frame is referred to as CaFAB1. The levels of identity to potential Fab1p homologues from the higher eukaryotic organisms Arabidopsis thaliana and Mus musculus were lower, 38 and 35%, respectively (41;Genbank accession no. AL035525).
The highest level of identity between Fab1 proteins was observed in the putative kinase domain (amino acids 2119 to 2253 in CaFab1p). The amino acids that are involved in kinase activity of ScFab1p were also found in CaFab1p (19). Another region of high homology between Fab proteins is the FYVE domain comprising amino acids 236 to 311 of the putative CaFab1 protein (Fig. 3). The crystal structure of the FYVE domain from Vps27 of S. cerevisiae indicates the cysteine residues that coordinate the Zn2+ atoms and the amino acids that participate in the putative PI(3)P binding pocket (9, 33). Most of these amino acids are conserved in CaFab1p; the only exception is the first cysteine, which is a leucine in CaFab1p at position 246 (Fig. 3). Additionally, a block search identified a region in the CaFab1p amino acid sequence that showed homology to a chloride channel motif (amino acids 1104 to 1111). A hydropathy plot of the deduced protein indicated that CaVps34p is highly hydrophilic (31).
FIG. 3.
Multiple alignment of the FYVE finger domain from Fab1p of C. albicans (CaFab1), S. cerevisiae (ScFab1), S. pombe (SpFab1), A. thaliana (AtFab1), and mouse (MmFab1), as well as the Vps27 protein from S. cerevisiae (ScVps27) and the Vac1 homologue EAA1 from humans (HuEEA1). Zn2+-binding cysteines are indicated by asterisks. Amino acids that are involved in PI binding are indicated by plus signs.
Disruption of CaFAB1.
To characterize the function of CaFAB1, null mutants were constructed, and their phenotypes were analyzed (see Materials and Methods). A 4.7-kb SacI/PstI fragment of pFD2 (Fig. 1) was used to transform C. albicans Ura− strain CAI-4. Southern analysis of XbaI-digested DNA from a representative Ura+ transformant (Fig. 2) resulted in two bands in the case of a heterozygous Cafab1 mutant (CAF1). The approximately 22.0-kb fragment represented the CaFAB1 wild-type allele, whereas the 10.2-kb fragment confirmed that a 6.7-kb fragment of one CaFAB1 allele had been replaced by the 4.1-kb hisG-URA3-hisG cassette since the integrated URA blaster contained two internal XbaI sites (lane 2). Excision of one copy of hisG and the URA3 gene in the Ura− derivatives resulted in a 8.9-kb band (CAF2) (lane 3). A second transformation with the same disruption construct led to isolation of a Cafab1 null mutant (CAF3). The loss of the approximately 22.0-kb fragment and the sizes of the remaining fragment are consistent with replacement of the second CaFAB1 allele (lane 4). Southern analysis of a representative Ura− segregant showed only a 8.9-kb hybridizing DNA fragment, which indicated that two hisG disrupted alleles were present (CAF4) (lane 5). After transformation of CAF4 with pFXU1 (Fig. 1), the reintegrated CaFAB1/URA3 cassette resulted in a 17.0-kb fragment because the CaURA3 fragment contained an XbaI site (CAF5) (Fig. 2, lane 6).
Disruption of CaFAB1 causes defects in growth, vacuole morphology, acidification, and chromosome transmission.
Yeast-phase growth of strains SC5314, CAF1, CAF3, and CAF5 was compared in Sabouraud medium at 30°C. We observed a difference in generation time between the null mutant strain and the wild-type, heterozygote mutant, and reintegration strains. The generation times were as follows: SC5314, 111 min; FAB1/Δfab1, 112 min; Δfab1/Δfab1, 123 min; and Δfab1/FAB1-URA3, 112 min. These values were confirmed in a second growth experiment.
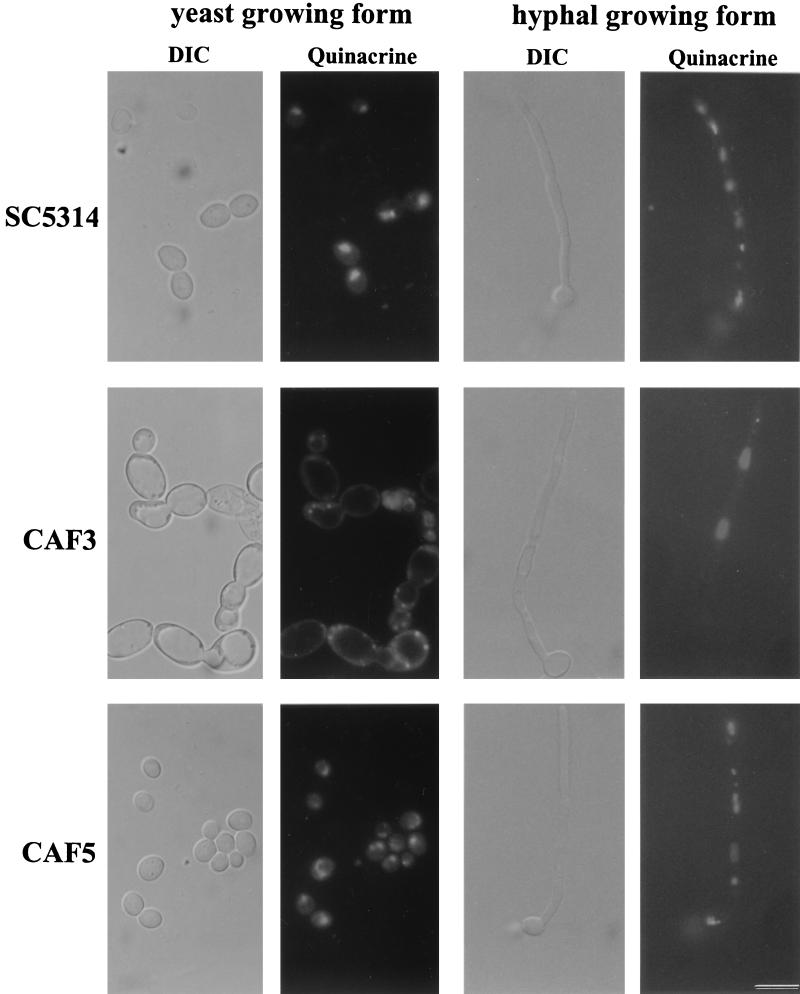
The vacuole morphologies of C. albicans wild-type strain SC5314, Cafab1 null mutant CAF3, and revertant strain CAF5 were first examined by light microscopy (differential interference contrast [DIC]) (Fig. 4). In C. albicans wild-type cells and in the revertant cells vacuoles appeared as several small organelles that occupied a small fraction of the total cell volume. Morphological examination of the Cafab1 null mutant cells, however, revealed enlarged vacuoles. In a population of cells giant vacuoles occupied approximately 80% of the total cell volume. The hyphal growing form of CAF3 also contained enlarged vacuoles in the mother cell and in the hyphae. Thus, the Cafab1 null mutation resulted in a substantial vacuole morphology defect. S. cerevisiae fab1 and the Cavps34 null mutant showed a strong acidification defect in their enlarged vacuoles (46). Therefore, we investigated vacuole acidification of the Cafab1 null mutant by staining with Quinacrine, a fluorescent weak base which accumulates in the vacuole and becomes protonated by the acidic environment. We observed clear vacuoles in the yeast and hyphal growing forms of CAF3, which thus showed an acidification defect. In the apical region of hyphae acidification was restored. The wild-type and the revertant cells contained fluorescent vacuoles that are typical of a normal acid vacuole lumen (Fig. 4).
FIG. 4.
Morphology of C. albicans fab1 null mutant CAF3, wild-type strain SC5314, and revertant strain CAF5. The use of Nomarski optics clearly revealed enlarged vacuoles and cells for CAF3 during yeast-phase growth compared to SC5314 and CAF5. The hyphal growing form of CAF3 also contains enlarged vacuoles in the mother cell and in the hyphae. Quinacrine staining revealed clear vacuoles in the yeast and hyphal growing forms of CAF3, indicating an acidification defect. In the apical regions of hyphae acidification was restored. In SC5314 and CAF5 we observed normally acidified vacuoles. Bar = 13 μm (for all panels).
The fab1 mutant of S. cerevisiae exhibited an abnormal chromosome distribution after a shift to 37°C. More than 20% of the cells became aploid or binucleate (46). Therefore, we examined this phenotype in the Cafab1 mutant CAF3 and compared it to the phenotype in the wild-type strain and CAF5. After the nuclei were stained by DAPI, 300 cells per preparation were analyzed. In the wild-type and in CAF5 we observed only 0.5% aploid cells and 99.5% cells with one nucleus at 28°C. The CAF3 culture contained 6% binucleate cells and 94% cells with one nucleus. When cells were shifted to 37°C for 2 h, no changes in the percentages of aploid and polynucleate cells were observed. In contrast to S. cerevisiae fab1, C. albicans fab1 (CAF3) exhibited no aploid cells.
In summary, the Cafab1 null mutant exhibited phenotypes which were also observed for S. cerevisiae fab1 cells (enlarged vacuoles, vacuolar acidification defect, formation of binucleate cells). This supports the assumption that we cloned a FAB1 homologous gene.
Cafab1 null mutant does not exhibit increased sensitivity to high-temperature and hyperosmotic stresses.
Recent analysis has shown that S. cerevisiae fab1 and vps34 strains, as well as the Cavps34 null mutant, are sensitive to temperature stress (6, 46). Since Fab1p in S. cerevisiae is a downstream effector of Vps34p that uses its product, PI(3)P, as a substrate, we supposed that this may be also true for the Cafab1 null mutant. Therefore, we tested the growth of C. albicans strains SC5314, CAF1, and CAF3 on YPD medium plates incubated at 30, 37, 40, 42, and 45°C. It was surprising to find that parental strain SC5314, heterozygous mutant strain CAF1, and null mutant strain CAF3 grew well at all temperatures tested.
VPS34 mutants of S. cerevisiae, Schizosaccharomyces pombe, and C. albicans are sensitive to hyperosmotic pressure (6, 23, 27). Furthermore, the S. cerevisiae fab1 strain appeared to be osmoremedial and Ca2+ sensitive (46). Therefore, we compared the abilities of C. albicans fab1 and SC5314 to grow on solid medium supplemented with different osmotic supports. After 3 days of incubation at 28 and 37°C, the growth of CAF3 did not differ from that of wild-type strain SC5314 in the presence of 0.5 or 1.5 M NaCl and in the presence of 0.5 or 1.5 M KCl, respectively. Hence, the CaFAB1 disruption did not result in increased sensitivity to osmotic stress or osmoremediation. Additionally, the Cafab1 null mutant was not sensitive to 0.5 M CaCl2.
CaFAB1 influences dimorphic growth of C. albicans under different conditions.
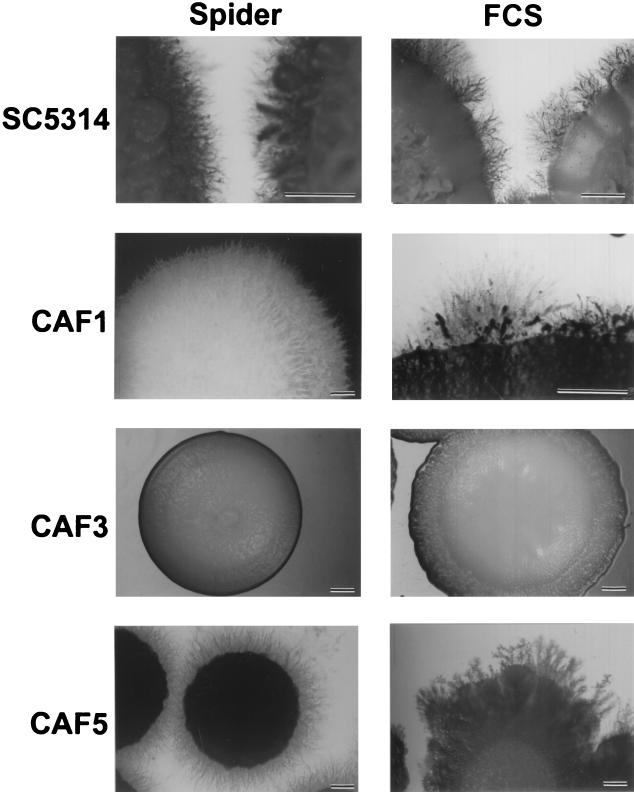
Dimorphism is considered an important virulence factor of C. albicans (32). Consequently, signal transduction cascades leading to morphogenetic changes are the subject of intense studies (5). Since the phosphatidylinositol 3-kinase (Vps34p) mutant showed delayed induction of hyphae in liquid media and there was complete inhibition of hyphal growth on different solid media, we investigated the yeast-to-hypha transition of the Cafab1 null mutant (6). Hyphal growth of C. albicans fab1 strain CAF3 was induced in YPD medium with 10% FCS at 37°C. We observed that induction of hyphae was not delayed compared to induction of hyphae in wild-type strain SC5314. Similar results were obtained for induction in liquid Spider medium at 37°C. Furthermore, we tested the Cafab1 mutant strains for hyphal induction on solid Spider medium containing mannitol as a carbon source at 37°C. As shown in Fig. 5, nutritional starvation was sufficient to induce hyphal differentiation in the wild-type strain but not in the mutant strain. Moreover, CAF3 also had completely lost the ability to form hyphae on YPD agar plates containing 10% FCS. In revertant strain CAF5 the wild-type phenotype was reconstituted (Fig. 5).
FIG. 5.
Phenotypes of Cafab1 mutant strains on solid hypha-inducing media. Spider medium contained mannitol as a carbon source. YPD medium contained 10% FCS. The strains used were C. albicans SC5314 (wild type), CAF1 (Cafab1 heterozygous mutant), CAF3 (Cafab1 homozygous mutant), and CAF5 (CaFAB1 revertant). The strains were grown for 7 days at 37°C. Bars = 3.0 mm.
Adhesion of the Cafab1 null mutant to epidermis-like HeLa cells is not decreased.
Adhesion of C. albicans to host epithelial and endothelial cells is thought to be a prerequisite for virulence. The avirulent Cavps34 null mutant showed strongly decreased adhesion to HeLa cells (A. Hartmann and R. Eck et al., unpublished data). Thus, it was interesting test the adhesion of Cafab1 null mutant CAF3 on HeLa cells. In two experiments mutant strain CAF3 showed 101% ± 14% and 140% ± 23% adhesion compared to the values for wild-type strain SC5314 (defined as 100% ± 9% and 100% ± 18%). Heterozygous mutant strain CAF1 exhibited 90% ± 8% and 98% ± 23% adhesion in two experiments. The results indicated that the adhesion of CAF3 to HeLa cells was not significantly different from the adhesion of wild-type strain SC5314 or strain CAF1 (P < 0.025).
CaFab1p activity is not required for virulence of C. albicans in a mouse model of systemic candidosis.
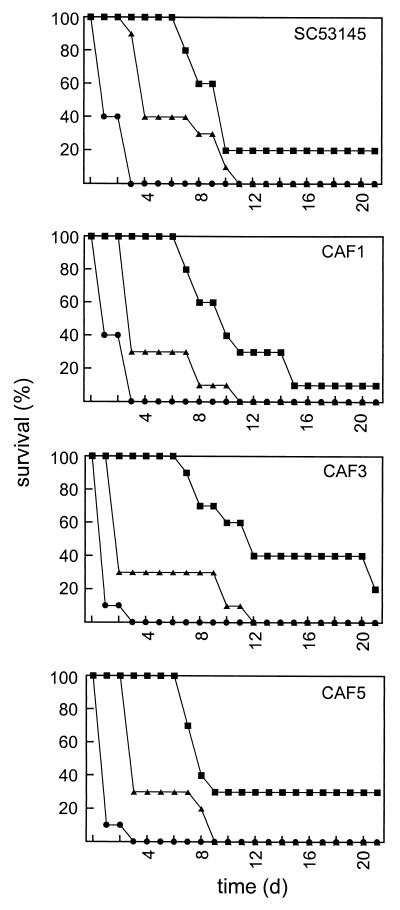
Since hyphal growth, which plays an important role in the pathogenesis of a C. albicans infection, is affected in the Cafab1 null mutant, we expected that disruption of CaFAB1 would have an effect on the virulence of C. albicans. Therefore, we tested the virulence of CAF1, the virulence of CAF3, and the virulence of CAF5 and compared the results to the results obtained with wild-type strain SC5314 in a mouse model of systemic candidosis (Fig. 6). However, we did not observe significantly reduced virulence of the heterozygous Cafab1 mutant, the null mutant, or the revertant (P < 0.05).
FIG. 6.
Pathogenicity of the Cafab1 mutants. C. albicans SC5314, CAF1 (FAB1/fab1::hisG-URA3-hisG), CAF3 (fab1::hisG-URA3-hisG/fab1::hisG), and CAF5 (fab1::hisG/FAB1-URA3) were tested in a mouse model of systemic candidosis. Survival of mice infected with 5 × 104 (▪), 5 × 105 (▴), and 5 × 106 (•) cells was monitored for 21 days (n = 10).
Systemic candidosis is often associated with colonization of internal organs, including the kidneys, the lungs, or the liver. It is known that in animal models of disseminated candidosis C. albicans exhibits a high predilection for the kidneys, which leads to late fatalities in the course of the infection (34). Three days after infection, we examined kidney colonization in four mice that were infected with 5 × 105 cells of strain SC5314, CAF3, or CAF5. All of the kidneys exhibited a high fungal burden (2.5 × 103 to 2.7 × 105 CFU per g of kidney tissue).
DISCUSSION
The FAB1 gene of C. albicans was isolated and sequenced. A 7,110-bp open reading frame coding for a predicted protein of 2,369 amino acids showed the highest homology to the FAB1 gene of S. cerevisiae, which codes for a PI(3)P 5-kinase. The highest identity between Fab1 proteins was observed in the putative kinase domain (amino acids 2119 to 2253 in CaFab1p). The GGXXG motif (amino acids 2133 to 2137 in CaFab1p) is reminiscent of the highly conserved GXGXXG motif present in the ATP binding site of many kinases. Changes of the second and last glycines to valine in the GGXXG glycine triad motif and a change of asparagine to arginine (amino acid 2134 in ScFab1p) in the phosphate transfer region influence the lipid kinase activity of Fab1p of S. cerevisiae (19).
Another region of high homology in Fab proteins is the FYVE domain (Fig. 3). Besides conservative amino acids responsible for binding of Zn2+ and PI(3)P, the CaFab1p sequence contains an additional 13-amino-acid spacer between the β3 and β4 sheets compared to the ScFab1p and SpFab1p sequences. This spacer was also found in Vac1p of S. cerevisiae, a homologue of human EEA1. Both proteins are involved in endocytic docking or fusion. Since PI(3)P is not required for membrane binding of the Vac1p FYVE domain, it seems possible that the spacer of Vac1p and CaFab1p permits interactions with unknown larger membrane molecules (8). Additionally, a block search identified a specific region in the CaFab1p amino acid sequence that showed homology to a chloride channel motif (amino acids 1104 to 1111). However, it seems unlikely that CaFab1p is a chloride channel protein since it is a hydrophilic protein without typical transmembrane domains. On the other hand, the cytosolic soluble protein pICln of Xenopus laevis was postulated to be a chloride channel protein (25). This protein is involved in the transduction of swelling cells that leads to the opening of chloride channels.
After recently reporting the influence of a phosphatidylinositol 3-kinase on the virulence of C. albicans (6), here we present evidence that the downstream PI(3)P 5-kinase Fab1p is not involved in the pathogenicity process. Intriguingly, in our mouse model of systemic infection a C. albicans fab1 null mutant was completely virulent compared to the wild-type strain. Thus, our earlier supposition that the avirulence of the Cavps34 null mutant may be connected with the function of PI(3)P as substrate for CaFab1p was not confirmed. In the course of our work, we have ascertained CaFAB1 functions with regard to possible effects on virulence determinants, such as dimorphic growth in liquid media and adhesion to host cells. Both processes were shown to be not affected by deletion of both alleles of CaFAB1. Furthermore, sensitivities to hyperosmotic pressure and high temperature are not changed in the Cafab1 null mutant, in contrast to the Cavps34 null mutant (6). In contrast to S. cerevisiae FAB1, CaFAB1 is not essential for growth at 37°C. Additionally, we did not find indications of cell wall defects like those found for S. cerevisiae fab1 cells (46). Cavps34 null mutant and S. cerevisiae fab1 cells contain abnormally large vacuoles with an acidification defect. Since CaFAB1 also has this phenotype, this defect may not be responsible for the increased hyperosmotic sensitivity and high-temperature sensitivity of the Cavps34 null mutant and the Ca2+ sensitivity, high-temperature sensitivity, and osmoremediation of S. cerevisiae fab1 cells.
The differences between Cavps34 and Cafab1 null mutant phenotypes indicate that CaVps34p does not influence the pathogenicity of C. albicans via the CaFab1p signaling pathway. Other functions of PI(3)P as a second messenger and/or of CaVps34p as a multifunctional protein seem to be connected with the virulence of C. albicans.
Deletion of both copies of FAB1 influences the morphogenetic transition from yeast cells to hyphae on both solid Spider medium and solid medium containing serum. This defect in morphogenesis may be connected with the abnormal vacuoles. Recently, studies indicated that an acidification defect in the vacuole can influence the intracellular pH (2, 28). The changed pH influences the ion currents, which may play an active role in morphogenesis and hyphal growth (20, 26). Furthermore, it was observed that vacuolation is connected with hyphal growth (21). We did not observe such vacuolation in hyphal growing cells. However, the defects in morphogenesis of CAF3 were not connected with changes in virulence, indicating that mutants with defective hyphal growth on solid media can nevertheless be virulent in the mouse model of systemic candidosis. Additionally, the growth defect of CAF3 indicated that a reduced growth rate does not cause automatically lower virulence.
In this study the PI(3)P 5-kinase gene CaFAB1 was cloned and deleted in C. albicans. The Cafab1 null mutant had phenotypes that were both similar to and different from the phenotypes of S. cerevisiae fab1 and C. albicans vps34 strains. The virulence of the Cafab1 null mutant indicated that the previously reported avirulence of the Cavps34 null mutant is not connected with CaFab1p signaling. Further investigation is needed to elucidate the functions of CaVps34p and CaFab1p.
Acknowledgments
We are greatly indebted to A. Hartmann and E. Franzl for technical assistance. We also thank B. Frais, U. Stöckel, and B. Weber for technical assistance with the virulence test. We thank H.-M. Dahse for providing HeLa cells and W. A. Fonzi for the gift of plasmid pMB-7 and C. albicans CAI-4. We thank A. Bruckmann for helpful discussions and critical reading of the manuscript.
M.A. and C.H. contributed equally to this study.
Editor: T. R. Kozel
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipmann. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Banta, L. M., J. S. Robinson, D. J. Klikowsky, and S. D. Emr. 1988. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J. Cell Biol. 107:1369-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeke, J. D., F. LaCroute, and G. R. Fink. 1984. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 197:345-346. [DOI] [PubMed] [Google Scholar]
- 4.Borg-von Zepelin, M., and T. Wagner. 1995. Fluorescence assay for the detection of adherent Candida yeasts to target cells in microtest plates. Mycoses 38:339-347. [DOI] [PubMed] [Google Scholar]
- 5.Brown, A. J. P., and N. A. R. Gow. 1999. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 7:333-338. [DOI] [PubMed] [Google Scholar]
- 6.Bruckmann, A., W. Künkel, A. Härtl, R. Wetzker, and R. Eck. 2000. A phosphatidylinositol 3-kinase of Candida albicans influences adhesion, filamentous growth, and virulence. Microbiology 146:2755-2764. [DOI] [PubMed] [Google Scholar]
- 7.Bruckmann, A., W. Künkel, K. Augsten, R. Wetzker, and R. Eck. 2001. The deletion of CaVPS34 in the human pathogenic yeast Candida albicans causes defects in vesicle-mediated protein sorting and nuclear segregation. Yeast 18:343-353. [DOI] [PubMed] [Google Scholar]
- 8.Burd, C. G., M. Peterson, C. R. Cowles, and S. D. Emr. 1997. A novel Sec18/NSF-dependent complex required for Golgi-to-endosome transport in yeast. Mol. Cell. Biol. 8:1089-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burd, C. G., and S. D. Emr. 1998. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol. Cell 2:157-162. [DOI] [PubMed] [Google Scholar]
- 10.Caldera, J. A., and R. A. Calderone. 1999. Identification of a putative response regulator two-component phosphorelay gene (CaSSK1) from Candida albicans. Yeast 15:1243-1254. [DOI] [PubMed] [Google Scholar]
- 11.Chun, K. T., and M. G. Goebl. 1996. The identification of transposon-tagged mutations in essential genes that affect cell morphology in Saccharomyces cerevisiae. Genetics 142:39-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutler, J. E. 1991. Putative virulence factors of Candida albicans. Annu. Rev. Microbiol. 45:187-218. [DOI] [PubMed] [Google Scholar]
- 13.DeCamilli, P., S. D. Emr, P. S. McPherson, and P. Novick. 1996. Phosphoinositides as regulators in membrane traffic. Science 271:1533-1539. [DOI] [PubMed] [Google Scholar]
- 14.Dove, S. K., F. T. Cooke, M. R. Douglas, L. G. Sayers, P. J. Parker, and R. H. Mitchell. 1997. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature 390:187-192. [DOI] [PubMed] [Google Scholar]
- 15.Eberhard, D. A., C. L. Cooper, M. G. Low, and R. W. Holz. 1990. Evidence that the inositol phospholipids are necessary for exocytosis. Biochem. J. 268:15-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eck, R., C. Bergmann, K. Ziegelbauer, W. Schönfeld, and W. Künkel. 1997. A neutral trehalase gene from Candida albicans: molecular cloning, characterization and disruption. Microbiology 143:3747-3756. [DOI] [PubMed] [Google Scholar]
- 17.Eck, R., A. Bruckmann, R. Wetzker, and W. Künkel. 2000. A phosphatidylinositol 3-kinase of Candida albicans: molecular cloning and characterization. Yeast 16:933-944. [DOI] [PubMed] [Google Scholar]
- 18.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gary, J. D., A. E. Wurmser, C. J. Bonangelino, L. S. Weisman, and S. D. Emr. 1998. Fab1p is essential for PI(3)P 5-kinase activity and the maintenance of vacuolar size and homeostasis. J. Cell Biol. 143:65-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gow, N. A. R. 1994. Growth and guidance of the fungal hypha. Microbiology 140:3193-3205. [DOI] [PubMed] [Google Scholar]
- 21.Gow, N. A. R., and W. D. Gooday. 1982. Vacuolation, branch production and linear growth of germ tubes of Candida albicans. J. Gen. Microbiol. 128:2195-2198. [DOI] [PubMed] [Google Scholar]
- 22.Henikoff, S., and J. G. Henikoff. 1994. Protein family classification based on searching a database of blocks. Genomics 19:97-107. [DOI] [PubMed] [Google Scholar]
- 23.Herman, P. K., and S. D. Emr. 1990. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol. Cell. Biol. 10:6742-6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hube, B., M. Monod, D. A. Schofield, A. J. P. Brown, and N. A. R. Gow. 1994. Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol. Microbiol. 14:87-99. [DOI] [PubMed] [Google Scholar]
- 25.Jensch, J. T. 1994. Molecular physiology of anion channels. Curr. Opin. Cell Biol. 6:600-606. [DOI] [PubMed] [Google Scholar]
- 26.Kaur, S., and P. Mishra. 1994. Differential increase in cytoplasmatic pH at bud and germ tube formation in Candida albicans: studies of a nongerminant variant. Can. J. Microbiol. 40:720-723. [DOI] [PubMed] [Google Scholar]
- 27.Kimura, K., S. Miyake, M. Makuuchi, R. Morita, T. Usui, M. Yoshida, S. Horinouchi, and Y. Fukui. 1995. Phosphatidylinositol 3-kinase in fission yeast: a possible role in stress responses. Biosci. Biotechnol. Biochem. 59:678-682. [DOI] [PubMed] [Google Scholar]
- 28.Klikonsky, D. J., P. K. Herman, and S. D. Emr. 1990. The fungal vacuole: composition, function, and biogenesis. Microbiol. Rev. 54:266-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Köhler, J. R., and G. R. Fink. 1996. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc. Natl. Acad. Sci. USA 93:13223-13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Künkel, W., D. Berger, S. Risch, and B. Wittmann-Bresinsky. 1992. Genetic instability of industrial strains of Penicillium chrysogenum. Appl. Microbiol. Biotechnol. 36:499-502. [DOI] [PubMed] [Google Scholar]
- 31.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 32.Lo, H.-J., J. R. Köhler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous Candida albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 33.Misra, S., and J. H. Hurley. 1999. Crystal structure of a phosphatidylinositol 3-phosphate-specific membrane-targeting motif, the FYVE domain of Vps27p. Cell 97:657-666. [DOI] [PubMed] [Google Scholar]
- 34.Odds, F. C. 1988. Candida and candidosis. A review and bibliography, 2nd ed. Bailliere-Tindall, London, United Kingdom.
- 35.Odds, F. C. 1994. Pathogenesis of Candida infections. J. Am. Acad. Dermatol. 31:S2-S5. [DOI] [PubMed] [Google Scholar]
- 36.Odorizzi, G., M. Babst, and S. D. Emr. 1998. Fab1p PI(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 95:847-858. [DOI] [PubMed] [Google Scholar]
- 37.Peto, R., M. C. Pike, P. Armitage, N. E. Breslow, D. R. Cox, S. V. Howard, N. Mantel, K. McPherson, J. Peto, and P. G. Smith. 1977. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br. J. Cancer 35:1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piper, R. C., A. A. Cooper, H. Yang, and T. H. Stevens. 1995. VPS27 controls vacuolar endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J. Cell Biol. 131:603-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 40.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shisheva, A., D. Sbrissa, and O. Ikonomov. 1999. Cloning, characterization, and expression of a novel Zn2+-binding FYVE finger-containing phosphoinoside kinase in insulin-sensitive cells. Mol. Cell. Biol. 19:623-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stenmark, H., and R. Aasland. 1999. FYVE-finger proteins—effectors of an inositol lipid. J. Cell Sci. 112:4175-4183. [DOI] [PubMed] [Google Scholar]
- 43.Swoboda, R. K., G. Bertram, S. Budge, N. A. R. Gow, G. W. Gooday, and A. J. P. Brown. 1995. Structure and regulation of the HSP90 gene from the pathogenic fungus Candida albicans. Infect. Immun. 63:4506-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toker, A., and L. C. Cantley. 1997. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 387:673-676. [DOI] [PubMed] [Google Scholar]
- 45.Weisman, L. S., and W. Wicker. 1992. Molecular characterization of VAC1, a gene required for vacuole inheritance and vacuole protein sorting. J. Biol. Chem. 267:618-623. [PubMed] [Google Scholar]
- 46.Yamamoto, A., D. B. DeWald, I. V. Boronenkov, R. A. Anderson, S. D. Emr, and D. Koshland. 1995. Novel PI(4)5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol. Biol. Cell 6:525-539. [DOI] [PMC free article] [PubMed] [Google Scholar]