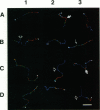
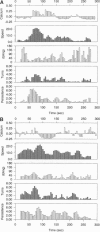
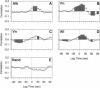
Abstract
The relationship between cytosolic free calcium concentration ([Ca2+]i) and human neutrophil motility was studied by video microscopy. Neutrophils stimulated by a uniform concentration of an N-formylated peptide chemoattractant (f-Met-Leu-Phe) were tracked during chemokinetic migration on albumin, fibronectin, and vitronectin. [Ca2+]i buffering with quin2 resulted in significant decreases in mean speed on albumin. To further characterize the relationship between [Ca2+]i changes and motility we carried out a cross-correlation analysis of [Ca2+]i with several motility parameters. Cross-correlations between [Ca2+]i and each cell's speed, angle changes, turn strength, and persistent forward motion revealed (i) a positive correlation between [Ca2+]i and cell speed (p < 0.05), (ii) no significant correlation between turns and calcium spikes, and (iii) the occurrence of turns during periods of low speed. Significant negative correlations between [Ca2+]i and angle change were noted on the high adhesion substrates vitronectin and fibronectin but not on the low adhesion substrate albumin. These data imply that there is a general temporal relationship between [Ca2+]i, speed, and persistent motion. However, the correlations are not sufficiently strong to imply that changes in [Ca2+]i are required proximal signals for velocity changes.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brundage R. A., Fogarty K. E., Tuft R. A., Fay F. S. Calcium gradients underlying polarization and chemotaxis of eosinophils. Science. 1991 Nov 1;254(5032):703–706. doi: 10.1126/science.1948048. [DOI] [PubMed] [Google Scholar]
- Brundage R. A., Fogarty K. E., Tuft R. A., Fay F. S. Chemotaxis of newt eosinophils: calcium regulation of chemotactic response. Am J Physiol. 1993 Dec;265(6 Pt 1):C1527–C1543. doi: 10.1152/ajpcell.1993.265.6.C1527. [DOI] [PubMed] [Google Scholar]
- Cassimeris L., Zigmond S. H. Chemoattractant stimulation of polymorphonuclear leucocyte locomotion. Semin Cell Biol. 1990 Apr;1(2):125–134. [PubMed] [Google Scholar]
- Caterina M. J., Devreotes P. N. Molecular insights into eukaryotic chemotaxis. FASEB J. 1991 Dec;5(15):3078–3085. [PubMed] [Google Scholar]
- Cembrowski G. S., Westgard J. O., Conover W. J., Toren E. C., Jr Statistical analysis of method comparison data. Testing normality. Am J Clin Pathol. 1979 Jul;72(1):21–26. doi: 10.1093/ajcp/72.1.21. [DOI] [PubMed] [Google Scholar]
- Chun J. S., Jacobson B. S. Spreading of HeLa cells on a collagen substratum requires a second messenger formed by the lipoxygenase metabolism of arachidonic acid released by collagen receptor clustering. Mol Biol Cell. 1992 May;3(5):481–492. doi: 10.1091/mbc.3.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J., Bresnick A., Demma M., Dharmawardhane S., Eddy R., Hall A. L., Sauterer R., Warren V. Mechanisms of amoeboid chemotaxis: an evaluation of the cortical expansion model. Dev Genet. 1990;11(5-6):333–340. doi: 10.1002/dvg.1020110504. [DOI] [PubMed] [Google Scholar]
- Cunningham C. C., Stossel T. P., Kwiatkowski D. J. Enhanced motility in NIH 3T3 fibroblasts that overexpress gelsolin. Science. 1991 Mar 8;251(4998):1233–1236. doi: 10.1126/science.1848726. [DOI] [PubMed] [Google Scholar]
- Devreotes P. N., Zigmond S. H. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- Dunn G. A., Brown A. F. A unified approach to analysing cell motility. J Cell Sci Suppl. 1987;8:81–102. doi: 10.1242/jcs.1987.supplement_8.5. [DOI] [PubMed] [Google Scholar]
- Dunn G. A., Zicha D. Long-term chemotaxis of neutrophils in stable gradients: preliminary evidence of periodic behavior. Blood Cells. 1993;19(1):25–41. [PubMed] [Google Scholar]
- Eliott S., Joss G. H., Spudich A., Williams K. L. Patterns in Dictyostelium discoideum: the role of myosin II in the transition from the unicellular to the multicellular phase. J Cell Sci. 1993 Feb;104(Pt 2):457–466. doi: 10.1242/jcs.104.2.457. [DOI] [PubMed] [Google Scholar]
- Foskett J. K. Simultaneous Nomarski and fluorescence imaging during video microscopy of cells. Am J Physiol. 1988 Oct;255(4 Pt 1):C566–C571. doi: 10.1152/ajpcell.1988.255.4.C566. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Lynch T. J., Brzeska H., Korn E. D. Myosin I is located at the leading edges of locomoting Dictyostelium amoebae. Nature. 1989 Sep 28;341(6240):328–331. doi: 10.1038/341328a0. [DOI] [PubMed] [Google Scholar]
- Giuliano K. A., Taylor D. L. Fluorescent actin analogs with a high affinity for profilin in vitro exhibit an enhanced gradient of assembly in living cells. J Cell Biol. 1994 Mar;124(6):971–983. doi: 10.1083/jcb.124.6.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick F., Meyer R., Stockem W. Visualization and measurement of calcium transients in Amoeba proteus by fura-2 fluorescence. Eur J Cell Biol. 1991 Aug;55(2):262–271. [PubMed] [Google Scholar]
- Gough A. H., Taylor D. L. Fluorescence anisotropy imaging microscopy maps calmodulin binding during cellular contraction and locomotion. J Cell Biol. 1993 Jun;121(5):1095–1107. doi: 10.1083/jcb.121.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruler H., Bültmann B. D. Analysis of cell movement. Blood Cells. 1984;10(1):61–77. [PubMed] [Google Scholar]
- Hall R. L., Peterson S. C. Trajectories of human granulocytes. Biophys J. 1979 Feb;25(2 Pt 1):365–372. doi: 10.1016/s0006-3495(79)85298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. S., Morrow J. S. Calmodulin and calcium-dependent protease I coordinately regulate the interaction of fodrin with actin. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3009–3013. doi: 10.1073/pnas.87.8.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., Thelen M., Rosen A., Janmey P. A., Nairn A. C., Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992 Apr 16;356(6370):618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Hendey B., Klee C. B., Maxfield F. R. Inhibition of neutrophil chemokinesis on vitronectin by inhibitors of calcineurin. Science. 1992 Oct 9;258(5080):296–299. doi: 10.1126/science.1384129. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jaconi M. E., Theler J. M., Schlegel W., Appel R. D., Wright S. D., Lew P. D. Multiple elevations of cytosolic-free Ca2+ in human neutrophils: initiation by adherence receptors of the integrin family. J Cell Biol. 1991 Mar;112(6):1249–1257. doi: 10.1083/jcb.112.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller H. U. Diacylglycerols and PMA are particularly effective stimulators of fluid pinocytosis in human neutrophils. J Cell Physiol. 1990 Dec;145(3):465–471. doi: 10.1002/jcp.1041450311. [DOI] [PubMed] [Google Scholar]
- Krause K. H., Demaurex N., Jaconi M., Lew D. P. Ion channels and receptor-mediated Ca2+ influx in neutrophil granulocytes. Blood Cells. 1993;19(1):165–175. [PubMed] [Google Scholar]
- Kruskal B. A., Shak S., Maxfield F. R. Spreading of human neutrophils is immediately preceded by a large increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986 May;83(9):2919–2923. doi: 10.1073/pnas.83.9.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Ishihara A., Jacobson K. How do cells move along surfaces? Trends Cell Biol. 1993 Nov;3(11):366–370. doi: 10.1016/0962-8924(93)90084-e. [DOI] [PubMed] [Google Scholar]
- Machesky L. M., Poland T. D. Profilin as a potential mediator of membrane-cytoskeleton communication. Trends Cell Biol. 1993 Nov;3(11):381–385. doi: 10.1016/0962-8924(93)90087-h. [DOI] [PubMed] [Google Scholar]
- Marks P. W., Hendey B., Maxfield F. R. Attachment to fibronectin or vitronectin makes human neutrophil migration sensitive to alterations in cytosolic free calcium concentration. J Cell Biol. 1991 Jan;112(1):149–158. doi: 10.1083/jcb.112.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. W., Kruskal B. A., Maxfield F. R. Simultaneous addition of EGF prolongs the increase in cytosolic free calcium seen in response to bradykinin in NRK-49F cells. J Cell Physiol. 1988 Sep;136(3):519–525. doi: 10.1002/jcp.1041360318. [DOI] [PubMed] [Google Scholar]
- Marks P. W., Maxfield F. R. Local and global changes in cytosolic free calcium in neutrophils during chemotaxis and phagocytosis. Cell Calcium. 1990 Feb-Mar;11(2-3):181–190. doi: 10.1016/0143-4160(90)90069-7. [DOI] [PubMed] [Google Scholar]
- Marks P. W., Maxfield F. R. Transient increases in cytosolic free calcium appear to be required for the migration of adherent human neutrophils. J Cell Biol. 1990 Jan;110(1):43–52. doi: 10.1083/jcb.110.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R. Regulation of leukocyte locomotion by Ca2+. Trends Cell Biol. 1993 Nov;3(11):386–391. doi: 10.1016/0962-8924(93)90088-i. [DOI] [PubMed] [Google Scholar]
- Murray J., Vawter-Hugart H., Voss E., Soll D. R. Three-dimensional motility cycle in leukocytes. Cell Motil Cytoskeleton. 1992;22(3):211–223. doi: 10.1002/cm.970220308. [DOI] [PubMed] [Google Scholar]
- Poenie M., Tsien R. Y., Schmitt-Verhulst A. M. Sequential activation and lethal hit measured by [Ca2+]i in individual cytolytic T cells and targets. EMBO J. 1987 Aug;6(8):2223–2232. doi: 10.1002/j.1460-2075.1987.tb02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratan R. R., Shelanski M. L., Maxfield F. R. Transition from metaphase to anaphase is accompanied by local changes in cytoplasmic free calcium in Pt K2 kidney epithelial cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5136–5140. doi: 10.1073/pnas.83.14.5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos F. J., Zimmermann A., Keller H. U. Effect of phorbol myristate acetate and the chemotactic peptide fNLPNTL on shape and movement of human neutrophils. J Cell Sci. 1987 Oct;88(Pt 3):399–406. doi: 10.1242/jcs.88.3.399. [DOI] [PubMed] [Google Scholar]
- Scanlon M., Williams D. A., Fay F. S. A Ca2+-insensitive form of fura-2 associated with polymorphonuclear leukocytes. Assessment and accurate Ca2+ measurement. J Biol Chem. 1987 May 5;262(13):6308–6312. [PubMed] [Google Scholar]
- Schwartz M. A. Transmembrane signalling by integrins. Trends Cell Biol. 1992 Oct;2(10):304–308. doi: 10.1016/0962-8924(92)90120-c. [DOI] [PubMed] [Google Scholar]
- Theriot J. A., Mitchison T. J. Actin microfilament dynamics in locomoting cells. Nature. 1991 Jul 11;352(6331):126–131. doi: 10.1038/352126a0. [DOI] [PubMed] [Google Scholar]
- Wessels D., Murray J., Jung G., Hammer J. A., 3rd, Soll D. R. Myosin IB null mutants of Dictyostelium exhibit abnormalities in motility. Cell Motil Cytoskeleton. 1991;20(4):301–315. doi: 10.1002/cm.970200406. [DOI] [PubMed] [Google Scholar]
- Wessels D., Soll D. R. Myosin II heavy chain null mutant of Dictyostelium exhibits defective intracellular particle movement. J Cell Biol. 1990 Sep;111(3):1137–1148. doi: 10.1083/jcb.111.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro D. J., Fluss S. R., Maxfield F. R. Acidification of endocytic vesicles by an ATP-dependent proton pump. J Cell Biol. 1983 Sep;97(3):929–934. doi: 10.1083/jcb.97.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]