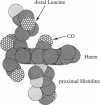
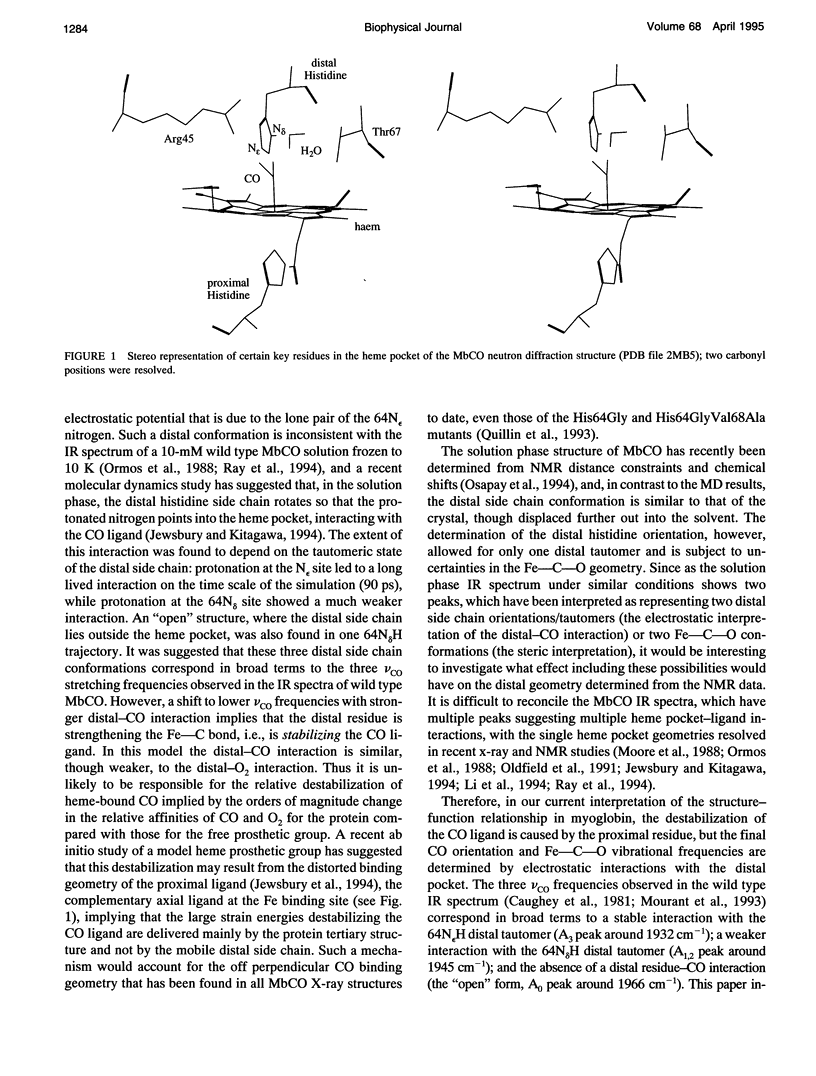
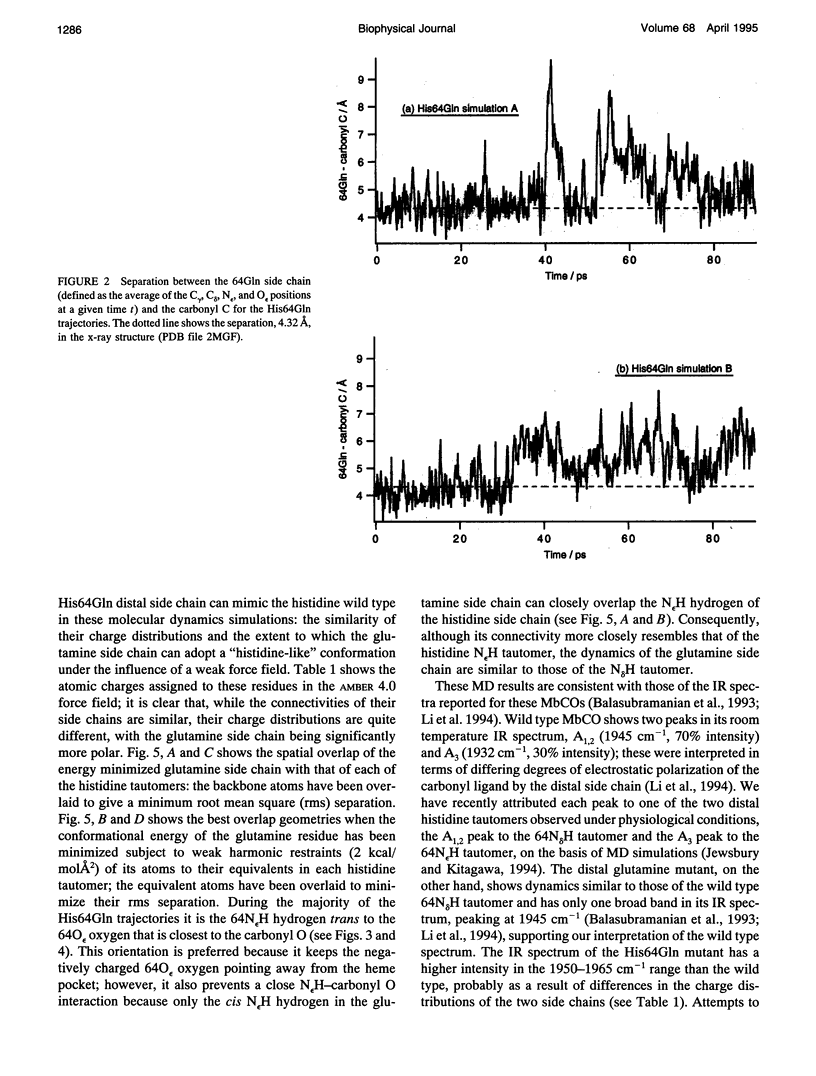
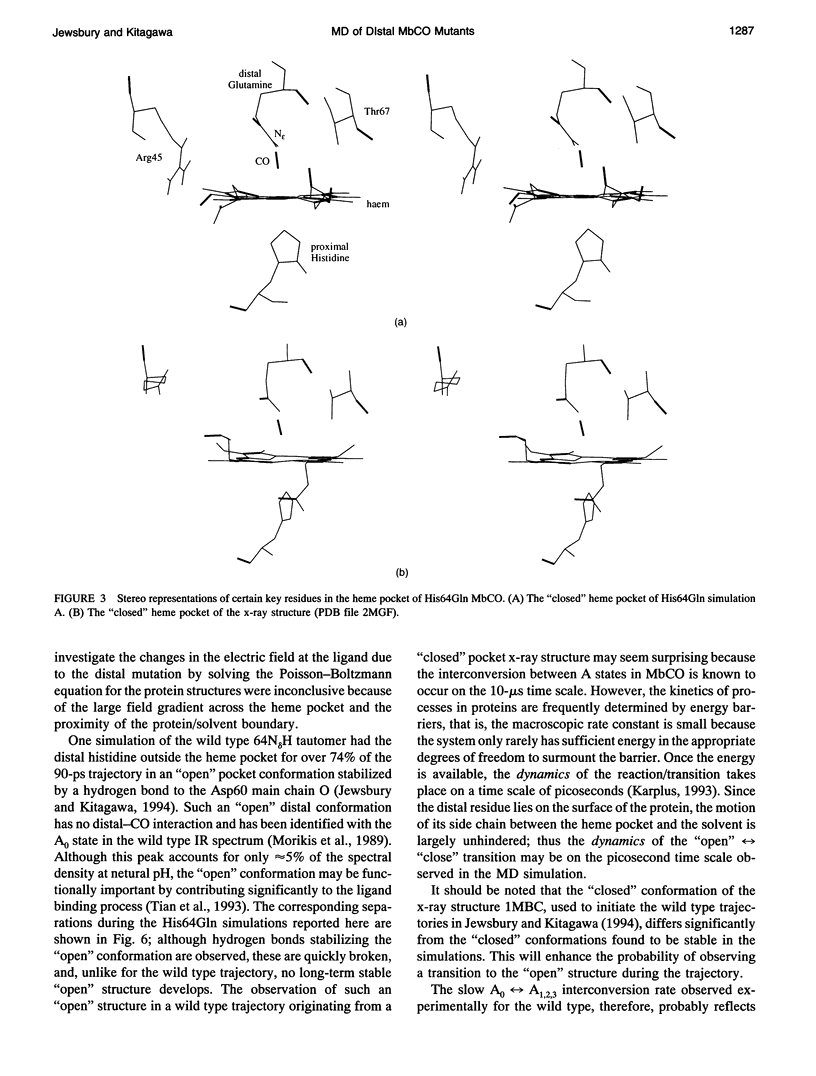
Abstract
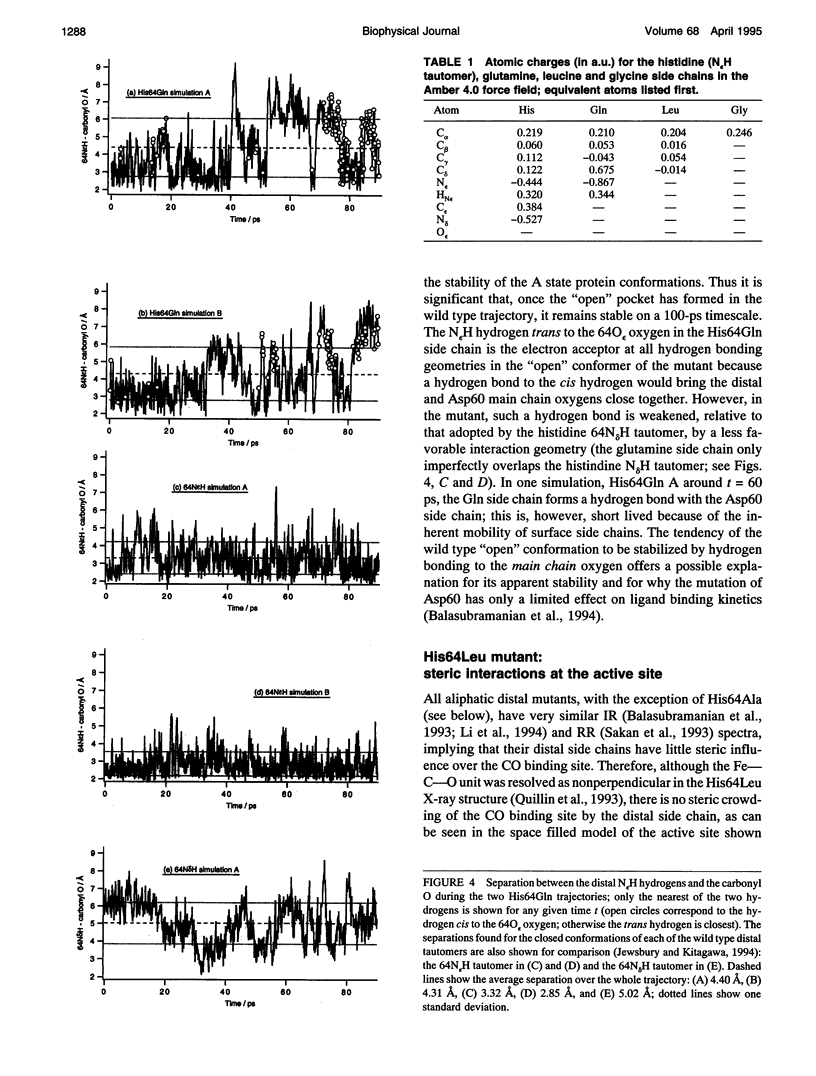
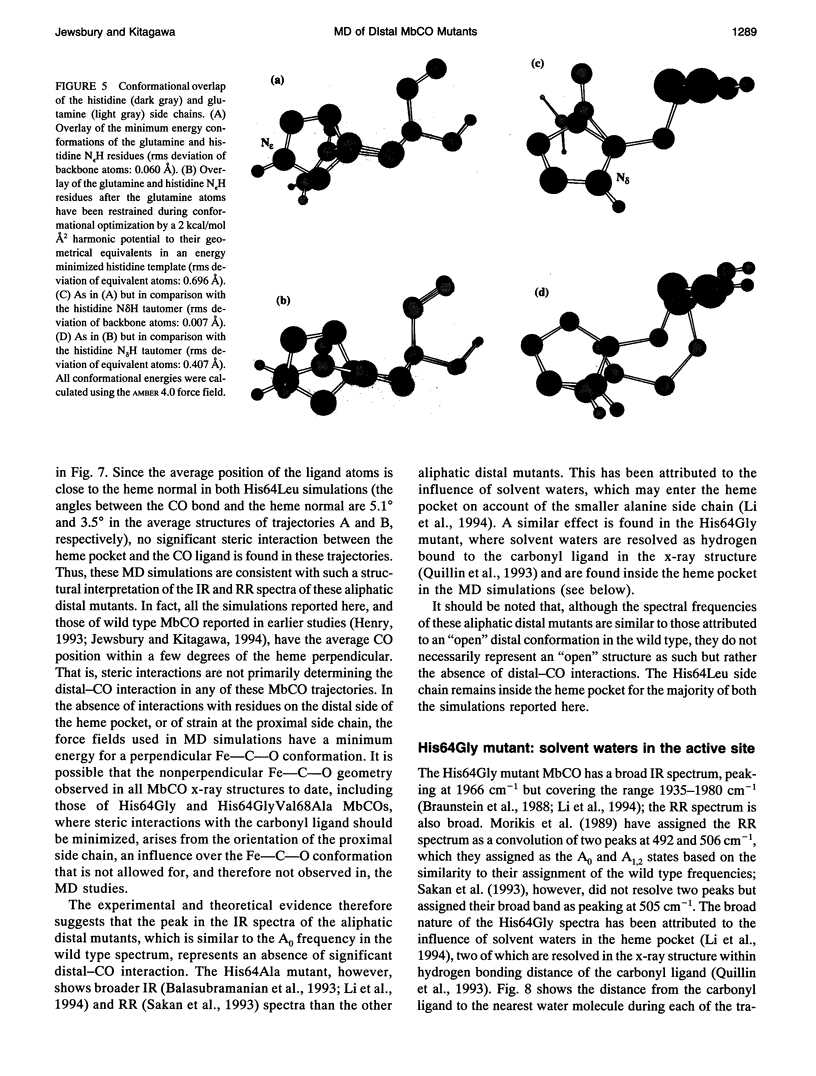
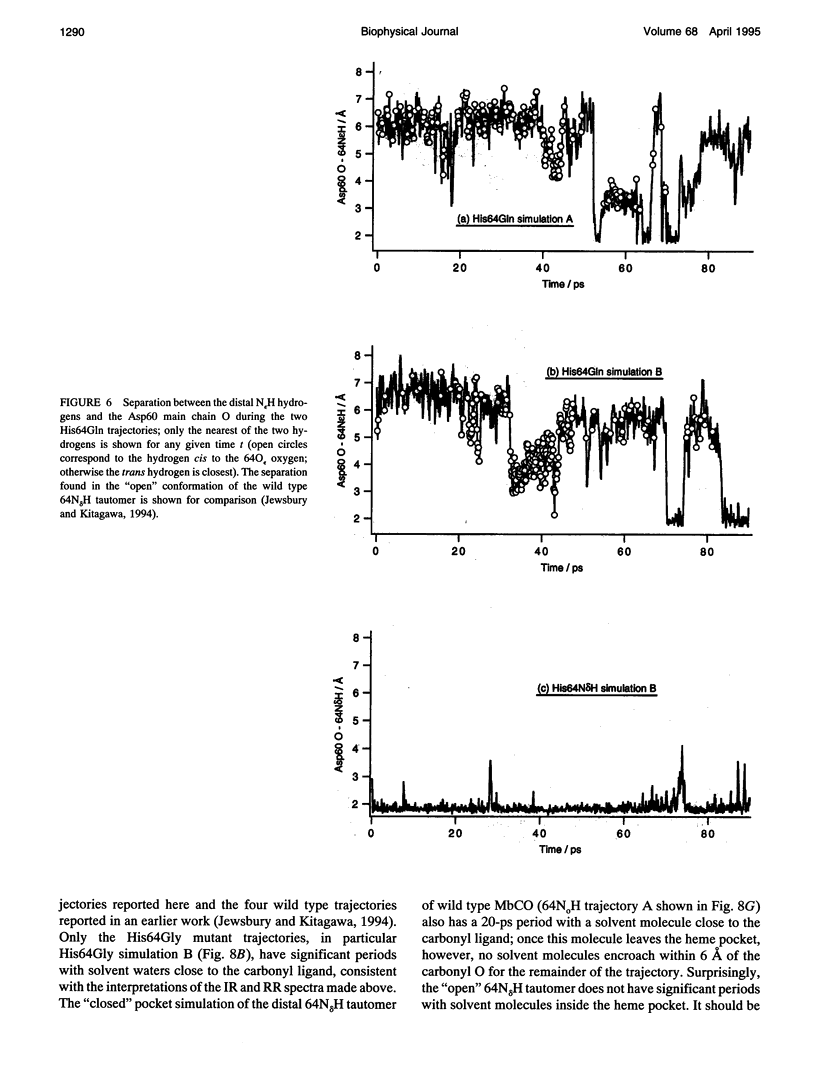
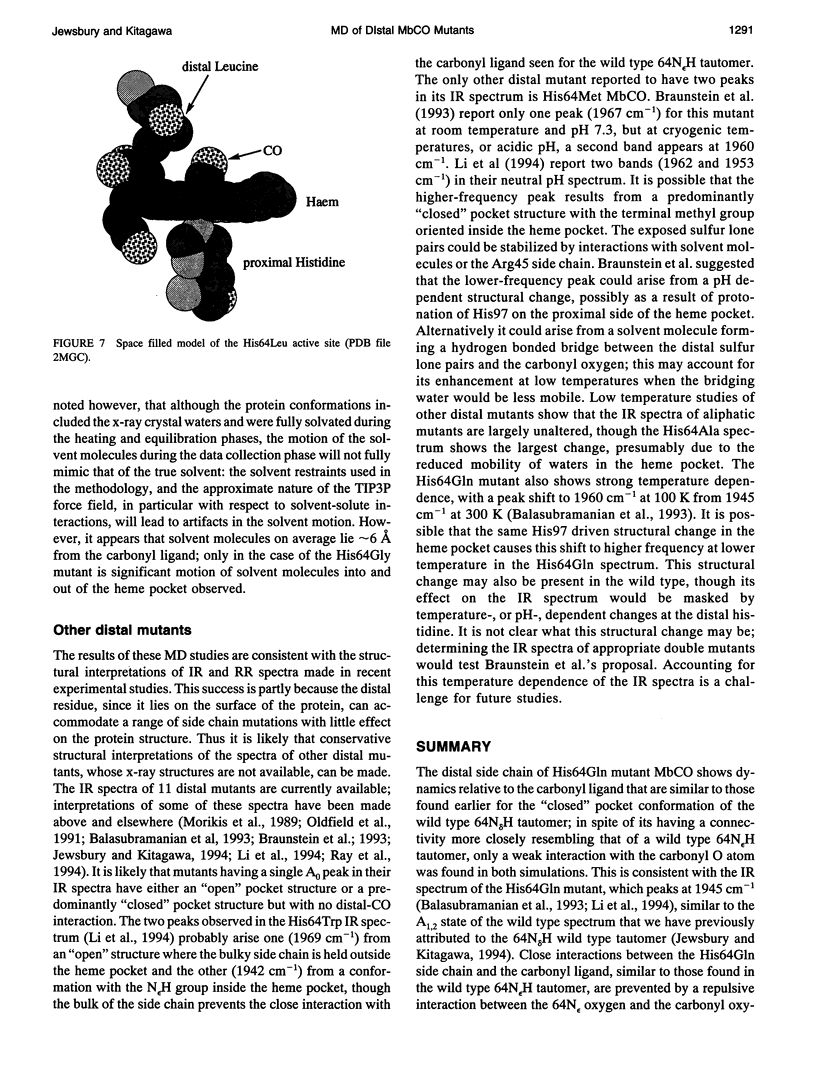
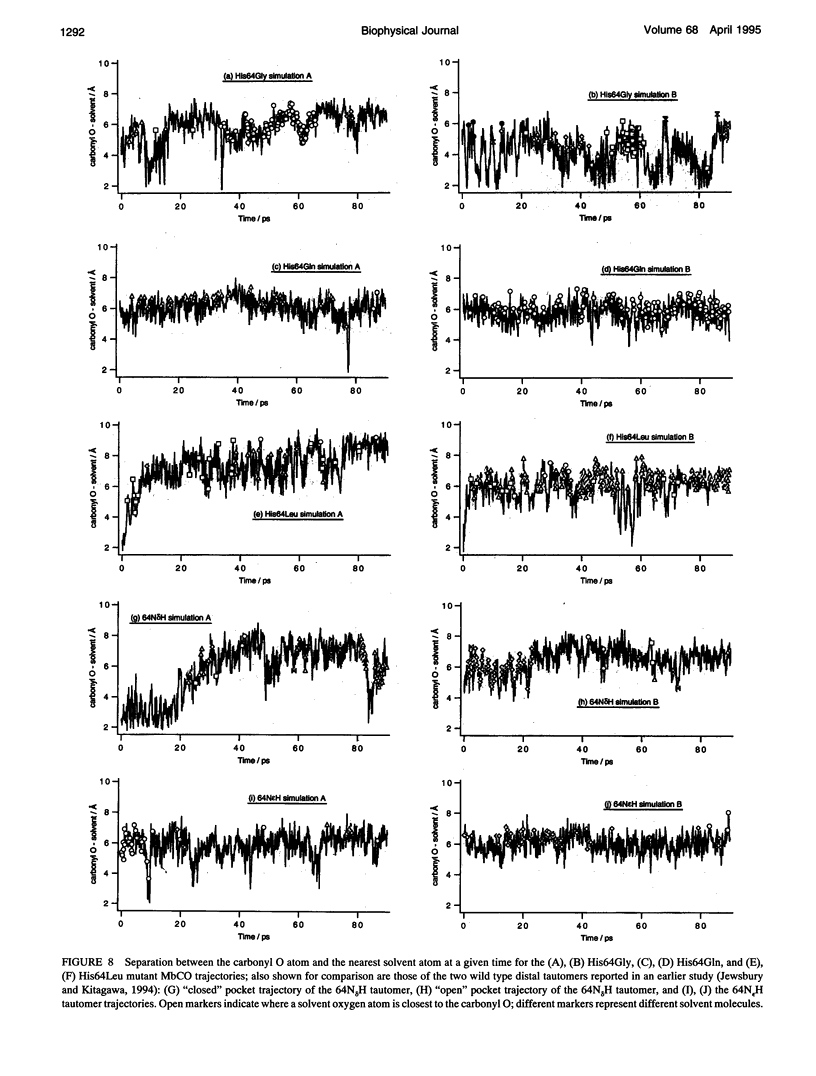
Six 90-ps molecular dynamics trajectories, two for each of three distal mutants of sperm whale carbonmonoxy myoglobin, are reported; solvent waters within 16 A of the active site have been included. In both His64GIn trajectories, the distal side chain remains part of the heme pocket, forming a "closed" conformation similar to that of the wild type 64N delta H tautomer. Despite a connectivity more closely resembling the N epsilon H histidine tautomer, close interactions with the carbonyl ligand similar to those observed for the wild type 64N epsilon H tautomer are prevented in this mutant by repulsive interactions between the carbonyl O and the 64O epsilon. The aliphatic distal side chain of the His64Leu mutant shows little interaction with the carbonyl ligand in either His64Leu trajectory. Solvent water molecules move into and out of the active site in the His64Gly mutant trajectories; during all the other carbonmonoxy myoglobin trajectories, including the wild type distal tautomers considered in an earlier work, solvent molecules rarely encroach closer than 6 A of the active site. These results are consistent with a recent structural interpretation of the wild type infrared spectrum, and the current reinterpretation that the distal-ligand interaction in carbonmonoxy myoglobin is largely electrostatic, not steric, in nature.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balasubramanian S., Lambright D. G., Boxer S. G. Perturbations of the distal heme pocket in human myoglobin mutants probed by infrared spectroscopy of bound CO: correlation with ligand binding kinetics. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4718–4722. doi: 10.1073/pnas.90.10.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S., Lambright D. G., Simmons J. H., Gill S. J., Boxer S. G. Determination of the carbon monoxide binding constants of myoglobin mutants: comparison of kinetic and equilibrium methods. Biochemistry. 1994 Jul 12;33(27):8355–8360. doi: 10.1021/bi00193a024. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Braunstein D. P., Chu K., Egeberg K. D., Frauenfelder H., Mourant J. R., Nienhaus G. U., Ormos P., Sligar S. G., Springer B. A., Young R. D. Ligand binding to heme proteins: III. FTIR studies of His-E7 and Val-E11 mutants of carbonmonoxymyoglobin. Biophys J. 1993 Dec;65(6):2447–2454. doi: 10.1016/S0006-3495(93)81310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein D., Ansari A., Berendzen J., Cowen B. R., Egeberg K. D., Frauenfelder H., Hong M. K., Ormos P., Sauke T. B., Scholl R. Ligand binding to synthetic mutant myoglobin (His-E7----Gly): role of the distal histidine. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8497–8501. doi: 10.1073/pnas.85.22.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case D. A., Karplus M. Stereochemistry of carbon monoxide binding to myoglobin and hemoglobin. J Mol Biol. 1978 Aug 25;123(4):697–701. doi: 10.1016/0022-2836(78)90214-0. [DOI] [PubMed] [Google Scholar]
- Caughey W. S., Shimada H., Choc M. G., Tucker M. P. Dynamic protein structures: infrared evidence for four discrete rapidly interconverting conformers at the carbon monoxide binding site of bovine heart myoglobin. Proc Natl Acad Sci U S A. 1981 May;78(5):2903–2907. doi: 10.1073/pnas.78.5.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X. D., Schoenborn B. P. Neutron diffraction study of carbonmonoxymyoglobin. J Mol Biol. 1991 Jul 20;220(2):381–399. doi: 10.1016/0022-2836(91)90020-7. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Brauman J. I., Halbert T. R., Suslick K. S. Nature of O2 and CO binding to metalloporphyrins and heme proteins. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3333–3337. doi: 10.1073/pnas.73.10.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry E. R. Molecular dynamics simulations of heme reorientational motions in myoglobin. Biophys J. 1993 Mar;64(3):869–885. doi: 10.1016/S0006-3495(93)81447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewsbury P., Kitagawa T. The distal residue-CO interaction in carbonmonoxy myoglobins: a molecular dynamics study of two distal histidine tautomers. Biophys J. 1994 Dec;67(6):2236–2250. doi: 10.1016/S0006-3495(94)80708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczera K., Kuriyan J., Karplus M. Temperature dependence of the structure and dynamics of myoglobin. A simulation approach. J Mol Biol. 1990 May 20;213(2):351–373. doi: 10.1016/S0022-2836(05)80196-2. [DOI] [PubMed] [Google Scholar]
- Kuriyan J., Wilz S., Karplus M., Petsko G. A. X-ray structure and refinement of carbon-monoxy (Fe II)-myoglobin at 1.5 A resolution. J Mol Biol. 1986 Nov 5;192(1):133–154. doi: 10.1016/0022-2836(86)90470-5. [DOI] [PubMed] [Google Scholar]
- Li T., Quillin M. L., Phillips G. N., Jr, Olson J. S. Structural determinants of the stretching frequency of CO bound to myoglobin. Biochemistry. 1994 Feb 15;33(6):1433–1446. doi: 10.1021/bi00172a021. [DOI] [PubMed] [Google Scholar]
- Moore J. N., Hansen P. A., Hochstrasser R. M. Iron-carbonyl bond geometries of carboxymyoglobin and carboxyhemoglobin in solution determined by picosecond time-resolved infrared spectroscopy. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5062–5066. doi: 10.1073/pnas.85.14.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikis D., Champion P. M., Springer B. A., Sligar S. G. Resonance raman investigations of site-directed mutants of myoglobin: effects of distal histidine replacement. Biochemistry. 1989 May 30;28(11):4791–4800. doi: 10.1021/bi00437a041. [DOI] [PubMed] [Google Scholar]
- Mourant J. R., Braunstein D. P., Chu K., Frauenfelder H., Nienhaus G. U., Ormos P., Young R. D. Ligand binding to heme proteins: II. Transitions in the heme pocket of myoglobin. Biophys J. 1993 Oct;65(4):1496–1507. doi: 10.1016/S0006-3495(93)81218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norvell J. C., Nunes A. C., Schoenborn B. P. Neutron diffraction analysis of myoglobin: structure of the carbon monoxide derivative. Science. 1975 Nov 7;190(4214):568–570. doi: 10.1126/science.1188354. [DOI] [PubMed] [Google Scholar]
- Ormos P., Braunstein D., Frauenfelder H., Hong M. K., Lin S. L., Sauke T. B., Young R. D. Orientation of carbon monoxide and structure-function relationship in carbonmonoxymyoglobin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8492–8496. doi: 10.1073/pnas.85.22.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osapay K., Theriault Y., Wright P. E., Case D. A. Solution structure of carbonmonoxy myoglobin determined from nuclear magnetic resonance distance and chemical shift constraints. J Mol Biol. 1994 Nov 25;244(2):183–197. doi: 10.1006/jmbi.1994.1718. [DOI] [PubMed] [Google Scholar]
- Phillips S. E., Schoenborn B. P. Neutron diffraction reveals oxygen-histidine hydrogen bond in oxymyoglobin. Nature. 1981 Jul 2;292(5818):81–82. doi: 10.1038/292081a0. [DOI] [PubMed] [Google Scholar]
- Quillin M. L., Arduini R. M., Olson J. S., Phillips G. N., Jr High-resolution crystal structures of distal histidine mutants of sperm whale myoglobin. J Mol Biol. 1993 Nov 5;234(1):140–155. doi: 10.1006/jmbi.1993.1569. [DOI] [PubMed] [Google Scholar]
- Sakan Y., Ogura T., Kitagawa T., Fraunfelter F. A., Mattera R., Ikeda-Saito M. Time-resolved resonance Raman study on the binding of carbon monoxide to recombinant human myoglobin and its distal histidine mutants. Biochemistry. 1993 Jun 8;32(22):5815–5824. doi: 10.1021/bi00073a014. [DOI] [PubMed] [Google Scholar]
- Tian W. D., Sage J. T., Champion P. M. Investigations of ligand association and dissociation rates in the "open" and "closed" states of myoglobin. J Mol Biol. 1993 Sep 5;233(1):155–166. doi: 10.1006/jmbi.1993.1491. [DOI] [PubMed] [Google Scholar]