Abstract
Orientia tsutsugamushi shows both pro- and antiapoptotic activities in infected vertebrate cells. Apoptosis of THP-1 cells induced by beauvericin was inhibited by O. tsutsugamushi infection. Beauvericin-induced calcium redistribution was significantly reduced and retarded in cells infected with O. tsutsugamushi. Antiapoptotic activities of O. tsutsugamushi in infected cells are most probably due to inhibition of the increase in the cytosolic calcium concentration.
Orientia tsutsugamushi, an obligate intracellular bacterium, is the causative agent of scrub typhus, which is endemic in many countries in the Asia-Pacific region, including Korea (3, 24). O. tsutsugamushi invades host cells by induced phagocytosis and escapes to the cytosol. Once free in the host cytoplasm, the bacteria replicate in the perinuclear area after movement by microtubules (15).
It has been shown previously that O. tsutsugamushi can induce apoptosis in a variety of host cells, including lymphocytes and endothelial cells (11, 12, 14). Many intracellular bacteria have evolved mechanisms by which to down-regulate apoptosis of the cells they infect (9). Apoptosis of the host cells would deprive the obligate intracellular pathogens of their intracellular hideouts, while apoptosis of infected macrophages or lymphocytes may seriously compromise the host defense mechanisms (22). Previous studies on the apoptosis of O. tsutsugamushi-infected cells (12, 14) have shown that apoptosis is a relatively late event and predominantly involves cells that are heavily laden with intracellular bacteria. The mechanisms of delayed apoptosis induced by O. tsutsugamushi have been elucidated in this study.
Cells.
The Boryong serotype of O. tsutsugamushi (3) was cultivated in ECV304 cells as described previously (14). When infected ECV304 cells showed a maximum cytopathic effect, the infected cells were disrupted with glass beads (diameter, 1.0 mm) and centrifuged at 300 × g for 5 min. The resulting supernatant, which was considered to consist of live O. tsutsugamushi, was used immediately to infect macrophages. Heat-killed O. tsutsugamushi was prepared by heating a bacterial preparation at 100°C for 5 min.
The human macrophage cell line THP-1 was obtained from the American Type Culture Collection (Manassas, Va.) and cultured in RPMI 1640 (Gibco BRL, Gaithersburg, Md.) supplemented with 10% fetal bovine serum (Gibco BRL) in humidified air with 5% CO2 at 37°C. Macrophages were grown in a 24-well culture plate until 90% of the bottom was filled with cells. Cells were treated with live O. tsutsugamushi, heat-killed O. tsutsugamushi, lipopolysaccharide (LPS; Sigma, St. Louis, Mo.), or fresh medium at day 0. Since LPS induces resistance to the apoptotic effects of various agents (10, 17), we always included LPS in all sets of experiments to exclude the confounding effect of contamination with LPS. One set of macrophages was stained for counting of bacteria by indirect immunofluorescence assay as described previously (14). At least 100 cells were counted at each time. Results were expressed as the mean number of O. tsutsugamushi organisms per cell. In most experiments, this value was about 15 after 3 h of infection. After 18 h, the cells were treated with various concentrations of beauvericin [cyclo(D-α-hydroxyisovaleryl-L-N-methyl-Phe)3] and staurosporine. The cells treated with beauvericin were harvested after 3 h of treatment.
Effect of O. tsutsugamushi on apoptosis induced by chemical inducers.
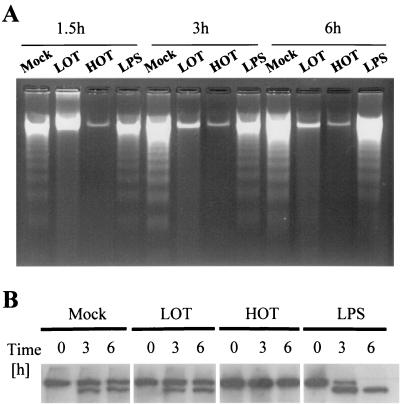
To investigate the effect of O. tsutsugamushi on host cell apoptosis, THP-1 cells were exposed to beauvericin and staurosporine. THP-1 cells were cultured in 24-well culture plates and incubated with either live or heat-killed O. tsutsugamushi for 18 h, and then apoptosis was induced. We isolated chromosomal DNA to assess the effect of O. tsutsugamushi on internucleosomal DNA fragmentation of THP-1 cells treated with beauvericin (Fig. 1A). The cells were harvested by centrifugation, washed with phosphate-buffered saline, resuspended in lysis buffer (10 mM Tris-HCl [pH 7.4], 10 mM NaCl, 10 mM EDTA, proteinase K at 0.1 mg/ml, 0.5% [wt/vol] sodium dodecyl sulfate), and incubated at 48°C overnight. DNA was precipitated with isopropanol and resuspended in TE buffer (10 mM Tris-HCl [pH 7.4], 1 mM EDTA). An aliquot of 20 μg of DNA from each sample was subjected to electrophoresis in a 1.5% agarose gel, and the DNA was stained with ethidium bromide. DNA fragmentation was not observed in THP-1 cells conditioned with live or heat-killed O. tsutsugamushi until 6 h following treatment with beauvericin. live and heat-killed O. tsutsugamushi also delayed the fragmentation of DNA of THP-1 cells that had been treated with staurosporine (data not shown). LPS did not inhibit DNA fragmentation. Inhibition of beauvericin-induced apoptosis was also evaluated by determining the PARP [poly(ADP-ribose)polymerase] cleavage pattern as described above. In the mock-treated group, PARP cleavage was evident from 3 h onward in THP-1 cells treated with beauvericin and about half of the PARP was degraded at 6 h. Heat-killed O. tsutsugamushi completely inhibited PARP cleavage induced by beauvericin. Live O. tsutsugamushi and LPS did not inhibit the cleavage of PARP (Fig. 1B).
FIG. 1.
(A) Inhibition of beauvericin-induced internucleosomal DNA fragmentation by O. tsutsugamushi infection. Chromosomal DNA from THP-1 cells was separated at various time points following treatment with beauvericin. (B) Immunoblotting of THP-1 cells with anti-PARP antibody at various time points after treatment with beauvericin. Lysates of THP-1 cells were separated by electrophoresis and stained with anti-PARP antibody. Cells were preconditioned by mock treatment or treatment with live O. tsutsugamushi (LOT) and heat-killed O. tsutsugamushi (HOT) 18 h before treatment with beauvericin.
Inhibition of apoptosis was confirmed by flow cytometric analysis with 7-amino-actinomycin D (7-AAD; Sigma) at 20 μg/ml as previously described (23). Samples were analyzed on a FACScalibur flow cytometer (Becton Dickinson, Mountain View, Calif.) equipped with a 15-mW air-cooled 488-nm argon laser. Ten thousand events were acquired for each analysis, and data were analyzed with CellQuest (Becton Dickinson) software.
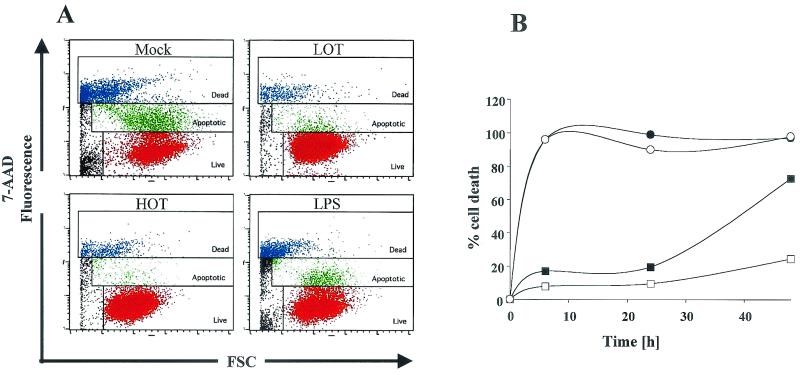
Flow cytometric analysis with 7-AAD staining revealed that THP-1 cells infected with O. tsutsugamushi significantly reduced apoptosis induced by beauvericin (Fig. 2A). In both mock-treated and LPS-treated cells, the extent of apoptosis induced by treatment with beauvericin began to increase from 1.5 h onward and became prominent at 3 h (data not shown). Fifty-five percent of mock-treated THP-1 cells underwent apoptosis 3 h after beauvericin treatment. At this time, the apoptotic fraction of THP-1 cells treated with beauvericin was less than 10% in the groups treated with live and heat-killed O. tsutsugamushi.
FIG. 2.
Antiapoptotic activity of O. tsutsugamushi on THP-1 cells undergoing apoptosis caused by chemical inducers. Both live O. tsutsugamushi (LOT) and heat-killed O. tsutsugamushi (HOT) show antiapoptotic activity against beauvericin- or staurosporine-induced apoptosis. (A) Flow cytometric analysis of apoptosis after staining of THP-1 cells with 7-AAD. Live cells were not stained with 7-AAD (red), apoptotic cells were stained weakly (green), and dead cells were stained brightly (blue). (B) Analysis of the duration of the antiapoptotic activity of O. tsutsugamushi for 48 h. Apoptosis of THP-1 cells was induced with beauvericin after they had been conditioned by mock treatment (closed circles) or treatment with LPS (open circles), live O. tsutsugamushi (closed rectangles), or heat-killed O. tsutsugamushi (open rectangles) for 18 h. FSC, forward scatter.
Since the THP-1 cells treated with heat-killed O. tsutsugamushi remained viable after beauvericin treatment, we proceeded to observe the duration of the antiapoptotic effect exerted by O. tsutsugamushi (Fig. 2B). THP-1 cells were incubated with 10 μM beauvericin for 48 h, and their viability was analyzed by Hoechst/propidium iodide staining. Virtually, all mock (closed circles)- or LPS (open circles)-treated THP-1 cells underwent apoptosis within 6 h. By contrast, proportions of viable cells in the heat-killed O. tsutsugamushi-treated cellular population (closed rectangles) at 24 and 48 h were 90.5 and 75.6%, respectively. The cells infected with live O. tsutsugamushi (open rectangles) were partially protected, with only 27.4% of the cells being viable 48 h following beauvericin treatment.
Effect on Ca2+ redistribution.
Since beauvericin induces apoptosis by releasing calcium from the endoplasmic reticulum (21), we examined the time course of increases in cytosolic intracellular free calcium in heat-killed O. tsutsugamushi-treated cells. By using fura-2 pentakis(acetoxymethyl) ester (fura 2-AM; Sigma) as a calcium indicator, we monitored the ratiometric (I340)/I380 fluorescence, which reflects the concentration of cytosolic free calcium ([Ca2+]in). After loading of fura 2-AM, cells were washed twice with normal Tyrode solution (5 mM HEPES [pH 7.4], 140 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 1 mM NaH2PO4, 5.5 mM glucose) and transferred to a glass bottom recording chamber on a Nikon Diaphot 300 epifluorescence microscope. Imaging of intracellular free Ca2+ was accomplished as follows. UV light from a xenon lamp (XBO-100) was filtered at two different wavelengths (340 and 380 nm) with band-pass filters (XF1002 and XF1003; Omega Optical, Inc., Brattleboro, Vt.). The wavelength of the excitation beam was changed with an optical filter changer (Lambda 10-2; Sutter Instrument, Novato, Calif.), and the beam was guided to an inverted epifluorescence microscope. Emitted light was collected with a cooled charge-coupled device camera system (PXL-37; Photometrics, Tucson, Ariz.) with an exposure time of 300 ms per single-wavelength image. The background-corrected fluorescence ratio of two excitation wavelengths (I340/I380) was calculated. A typical microscopic view through a 40× objective lens contains around 100 cells. A personal-computer-based imaging program (Axon Imaging Workbench; Axon Instruments, Inc., Union City, Calif.) was used to obtain and analyze data. Since the I340/I380 ratio represents [Ca2+]in, we did not attempt to calibrate and convert the ratio into [Ca2+]in.
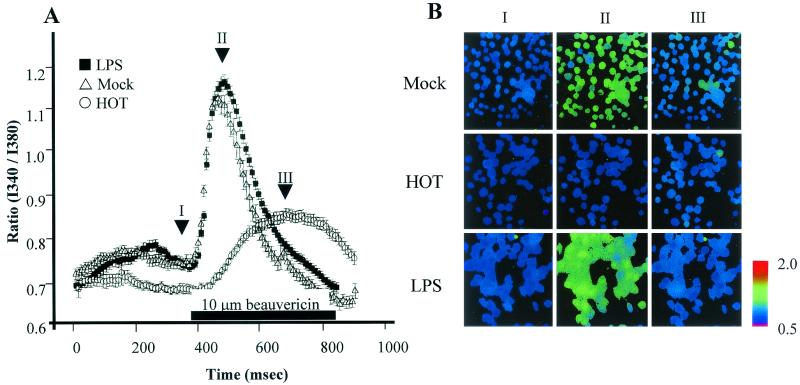
We first superfused a Ca2+-free solution and waited at least 6 min until [Ca2+]in became stable to observe release of calcium from the endoplasmic reticulum by beauvericin. The I340/I380 ratio measured at 6 min was 0.749 ± 0.01 in mock-treated THP-1 cells, 0.745 ± 0.01 in LPS-treated cells, and 0.69 ± 0.01 in heat-killed O. tsutsugamushi-treated cells (Fig. 3A). With the addition of 10 μM beauvericin, this ratio increased in all three groups. By 110 s, this ratio peaked at 1.11 ± 0.02 and 1.17 ± 0.01 in the mock (open triangles)- and LPS (closed triangles)-treated groups, respectively. On the other hand, the I340/I380 ratio of THP-1 cells treated with heat-killed O. tsutsugamushi increased slowly and the changes were comparable to those observed in mock-treated cells. Figure 3B is a frame-by-frame comparison of three groups at three different time points, as indicated by the arrows in Fig. 3A (points I, II, and III). At point II, intracellular calcium was well visualized in mock-treated or LPS-treated THP-1 cells. No intracellular calcium was observed in heat-killed O. tsutsugamushi-treated THP-1 cells at any point. Despite sustained beauvericin treatment, the ratio returned to a level similar to or slightly lower than the resting level.
FIG. 3.
Inhibition of increased cytosolic Ca2+ concentration by O. tsutsugamushi. (A) Fluorescence ratios of cells mock treated or treated with heat-killed O. tsutsugamushi (HOT) or LPS are plotted as a function of time. Ten micromolar beauvericin in Ca2+-free Tyrode solution was introduced at the time indicated by the bar. (B) The cells of the three groups were pseudocolored in accordance with the ratios at the three time points indicated by arrows in panel A.
Several studies have shown that O. tsutsugamushi has proapoptotic activity (6, 11, 12, 14). Although the mechanism of this proapoptotic activity is unknown, antiapoptosis at an early stage of infection and proapoptosis at a late stage of intracytoplasmic replication might be essential for the spread of O. tsutsugamushi (12, 14). The present report is probably the first to present data that suggest that O. tsutsugamushi can exert antiapoptotic activity on the macrophages it infects. Inhibition of host cell apoptosis by bacterial pathogens has been reported in several intracellular pathogens, as well as in viruses (2, 7, 8, 16). Some bacteria inhibit apoptosis mainly by inducing apoptosis-modulating cytokines such as interleukin-10 and tumor necrosis factor alpha or their decoy receptors (2, 16). Others inhibit apoptosis of host cells either by NF-κB activation or by other, hitherto unknown, processes dampening signal transduction pathways leading to apoptosis (6, 10). Yersinia YopJ/P represses the activation of NF-κB by inhibiting phosphorylation and subsequent degradation of its inhibitor protein, IκB (19). By contrast, R. rickettsii inhibits apoptosis by activation of the NF-κB signaling pathway (7). O. tsutsugamushi activates NF-κB in endothelial cells, as well as in macrophages (4, 5). Therefore, we evaluated whether NF-κB activation is essential for the antiapoptotic activity of O. tsutsugamushi. We included LPS, a strong activator of NF-κB, in all of our sets of experiments, but LPS did not prevent beauvericin- and staurosporine-induced apoptosis. Furthermore, inhibition of NF-κB activation with N-P-tosyl-l-phenylalanine chloromethyl ketone did not enhance the apoptosis of THP-1 cells infected with O. tsutsugamushi, suggesting that NF-κB activation by O. tsutsugamushi did not contribute to the antiapoptotic activity of O. tsutsugamushi (data not shown).
We have observed a marked alteration in the concentration and distribution of cytosolic Ca2+ in THP-1 cells treated with beauvericin as previously reported (21). The marked elevation of Ca2+ is a major triggering event in initiation of the apoptotic process (18, 20). The increased intracellular Ca2+ can initiate apoptosis by activating calcium-dependent endonuclease. Our data demonstrated a marked retardation of beauvericin-driven intracellular mobilization of Ca2+ in THP-1 cells treated with heat-killed O. tsutsugamushi. This probably explains the resistance to apoptosis of THP-1 cells infected with O. tsutsugamushi.
In our previous paper, we reported that a heat-stable molecule is responsible for the induction of cytokine production and that O. tsutsugamushi has mechanisms that suppress the production of inflammatory cytokines induced by its own heat-stable molecule (13). It is also interesting that heat-stable molecules induce a subset of chemokine genes in murine macrophages and human endothelial cells and that proliferation of O. tsutsugamushi is not a prerequisite for this action (4, 5). Therefore, it has been suggested that the biologically active components of O. tsutsugamushi are heat-stable molecules, such as lipids or polysaccharides. This is a rather surprising finding because O. tsutsugamushi has been reported to have neither LPS nor peptidoglycan (1). Taken together, our results suggest that heat-stable molecules of O. tsutsugamushi are responsible for the inhibition of chemically induced apoptosis and that heat-sensitive molecules produced by live O. tsutsugamushi suppress the activity of heat-stable molecules. Alternatively, It can also be postulated that host cells proceed to apoptosis when they sense stress levels greater than a predetermined threshold. Although heat-killed O. tsutsugamushi cannot proliferate inside cells, live O. tsutsugamushi can replicate inside cells. The increased number of pathogens inside cells elevates the level of stress imposed on cells. In this situation, the proapoptotic potential of O. tsutsugamushi exceeds the antiapoptotic activity that is present in O. tsutsugamushi intrinsically. This hypothesis may explain the antiapoptotic activity of O. tsutsugamushi in the early phase of infection and its proapoptotic activity in the late phase of infection. Because O. tsutsugamushi is an obligate intracellular bacterium that needs healthy cells for its proliferation, it may have developed some regulatory mechanisms by which to control the activity of its own components.
Further unraveling of the molecular and biochemical aspects of the bacterial factors that modulate apoptosis will not only increase our understanding of O. tsutsugamushi pathogenesis but also provide medical scientists with tools with which to dissect the molecular physiology of eukaryotic cells.
Acknowledgments
This work was supported by a grant from the Korea Health 21 R & D Project, Ministry of Health and Welfare, Seoul, Republic of Korea (01-PJ1-PG3-20200-0014).
Editor: A. D. O'Brien
REFERENCES
- 1.Amano, K., A. Tamura, N. Ohashi, H. Urakami, S. Kaya, and K. Fukushi. 1987. Deficiency of peptidoglycan and lipopolysaccharide components in Rickettsia tsutsugamushi. Infect. Immun. 55:2290-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balcewicz-Sablinska, M. K., J. Keane, H. Kornfeld, and H. G. Remold. 1998. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-α. J. Immunol. 161:2636-2641. [PubMed] [Google Scholar]
- 3.Chang, W. H., J. S. Kang, W. K. Lee, M. S. Choi, and J. H. Lee. 1990. Serological classification by monoclonal antibodies of Rickettsia tsutsugamushi isolated in Korea. J. Clin. Microbiol. 28:685-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho, N. H., S. Y. Seong, M. S. Choi, and I. S. Kim. 2001. Expression of chemokine genes in human dermal microvascular endothelial cell lines infected with Orientia tsutsugamushi. Infect. Immun. 69:1265-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho, N. H., S. Y. Seong, M. S. Huh, T. H. Han, Y. S. Koh, M. S. Choi, and I. S. Kim. 2000. Expression of chemokine genes in murine macrophages infected with Orientia tsutsugamushi. Infect. Immun. 68:594-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi, N. J., M. K. Kim, H. J. Park, B. U. Lim, and J. S. Kang. 1998. Apoptosis of murine macrophage-like cells infected with Orientia tsutsugamushi. J. Korean Soc. Microbiol. 33:399-406. [Google Scholar]
- 7.Clifton, D. R., R. A. Goss, S. K. Sahni, D. van Antwerp, R. B. Baggs, V. J. Marder, D. J. Silverman, and L. A. Sporn. 1998. NF-κB-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc. Natl. Acad. Sci. USA 95:4646-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan, T., H. Lu, H. Hu, L. Shi, G. A. McClarty, D. M. Nance, A. H. Greenberg, and G. Zhong. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J. Exp. Med. 187:487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao, L. Y., and Y. A. Kwaik. 2000. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol. 8:306-313. [DOI] [PubMed] [Google Scholar]
- 10.Hu, X., E. Yee, J. M. Harlan, F. Wong, and A. Karsan. 1998. Lipopolysaccharide induces the antiapoptotic molecules, A1 and A20, in microvascular endothelial cells. Blood 92:2759-2765. [PubMed] [Google Scholar]
- 11.Kasuya, S., I. Nagano, T. Ikeda, C. Goto, K. Shimokawa, and Y. Takahashi. 1996. Apoptosis of lymphocytes in mice induced by infection with Rickettsia tsutsugamushi. Infect. Immun. 64:3937-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kee, S. H., K. A. Cho, M. K. Kim, B. U. Lim, W. H. Chang, and J. S. Kang. 1999. Disassembly of focal adhesions during apoptosis of endothelial cell line ECV304 infected with Orientia tsutsugamushi. Microb. Pathog. 27:265-271. [DOI] [PubMed] [Google Scholar]
- 13.Kim, M. K., and J. S. Kang. 2001. Orientia tsutsugamushi suppresses the production of inflammatory cytokines induced by its own heat-stable component in murine macrophages. Microb. Pathog. 31:145-150. [DOI] [PubMed] [Google Scholar]
- 14.Kim, M. K., S. H. Kee, K. A. Cho, M. H. Chung, B. U. Lim, W. H. Chang, and J. S. Kang. 1999. Apoptosis of endothelial cell line ECV304 persistently infected with Orientia tsutsugamushi. Microbiol. Immunol. 43:751-757. [DOI] [PubMed] [Google Scholar]
- 15.Kim, S. W., K. S. Ihn, S. H. Han, S. Y. Seong, I. S. Kim, and M. S. Choi. 2001. Microtubule- and dynein-mediated movement of Orientia tsutsugamushi to the microtubule organizing center. Infect. Immun. 69:494-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kremer, L., J. Estaquier, E. Brandt, J. C. Ameisen, and C. Locht. 1997. Mycobacterium bovis bacillus Calmette Guerin infection prevents apoptosis of resting human monocytes. Eur. J. Immunol. 27:2450-2456. [DOI] [PubMed] [Google Scholar]
- 17.Manna, S. K., and B. B. Aggarwal. 1999. Lipopolysaccharide inhibits TNF-induced apoptosis: role of nuclear factor-κB activation and reactive oxygen intermediates. J. Immunol. 162:1510-1518. [PubMed] [Google Scholar]
- 18.McConkey, D. J., and S. Orrenius. 1997. The role of calcium in the regulation of apoptosis. Biochem. Biophys. Res. Commun. 239:357-366. [DOI] [PubMed] [Google Scholar]
- 19.Monack, D. M., J. Mecsas, D. Bouley, and S. Falkow. 1998. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 188:2127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicotera, P., and S. Orrenius. 1998. The role of calcium in apoptosis. Cell Calcium 23:173-180. [DOI] [PubMed] [Google Scholar]
- 21.Ojcius, D. M., A. Zychlinsky, L. M. Zheng, and J. D. Young. 1991. Ionophore-induced apoptosis: role of DNA fragmentation and calcium fluxes. Exp. Cell Res. 197:43-49. [DOI] [PubMed] [Google Scholar]
- 22.Okada, H., F. Kobune, T. A. Sato, T. Kohama, Y. Takeuchi, T. Abe, N. Takayama, T. Tsuchiya, and M. Tashiro. 2000. Extensive lymphopenia due to apoptosis of uninfected lymphocytes in acute measles patients. Arch. Virol. 145:905-920. [DOI] [PubMed] [Google Scholar]
- 23.Philpott, N. J., A. J. Turner, J. Scopes, M. Westby, J. C. Marsh, E. C. Gordon-Smith, A. G. Dalgleish, and F. M. Gibson. 1996. The use of 7-amino actinomycin D in identifying apoptosis: simplicity of use and broad spectrum of application compared with other techniques. Blood 87:2244-2251. [PubMed] [Google Scholar]
- 24.Seong, S. Y., M. S. Choi, and I. S. Kim. 2001. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect. 3:11-21. [DOI] [PubMed] [Google Scholar]