Abstract
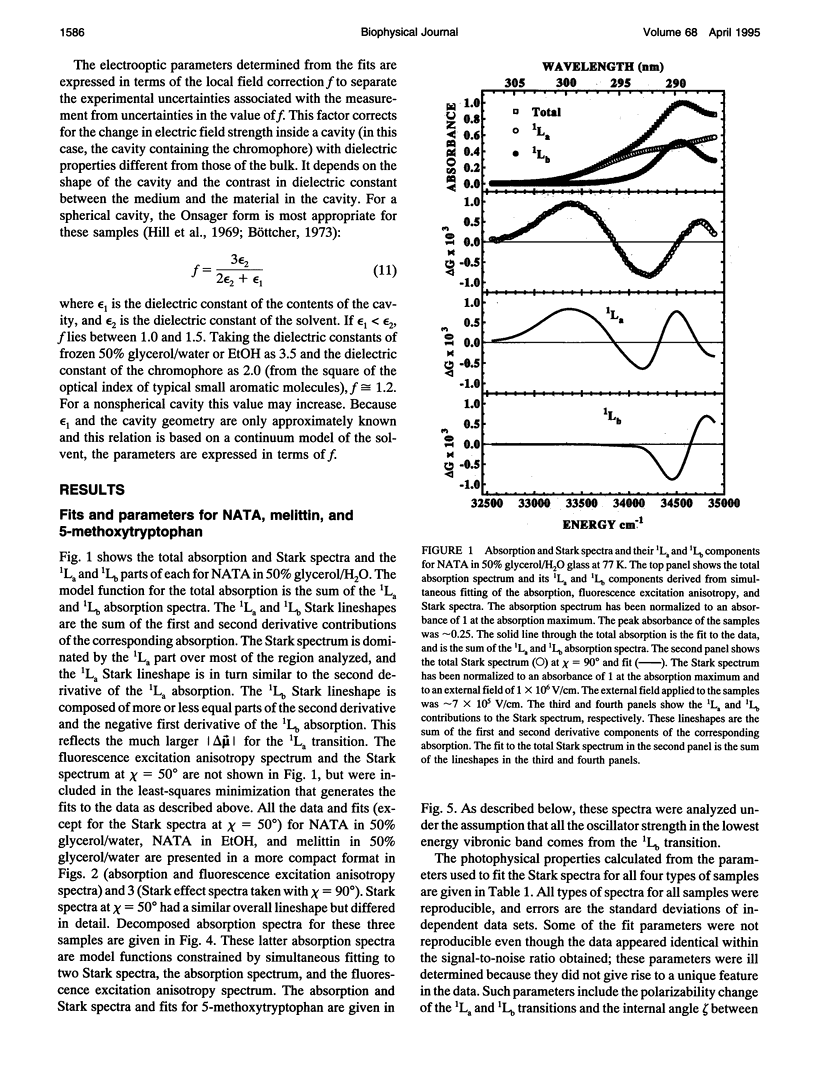
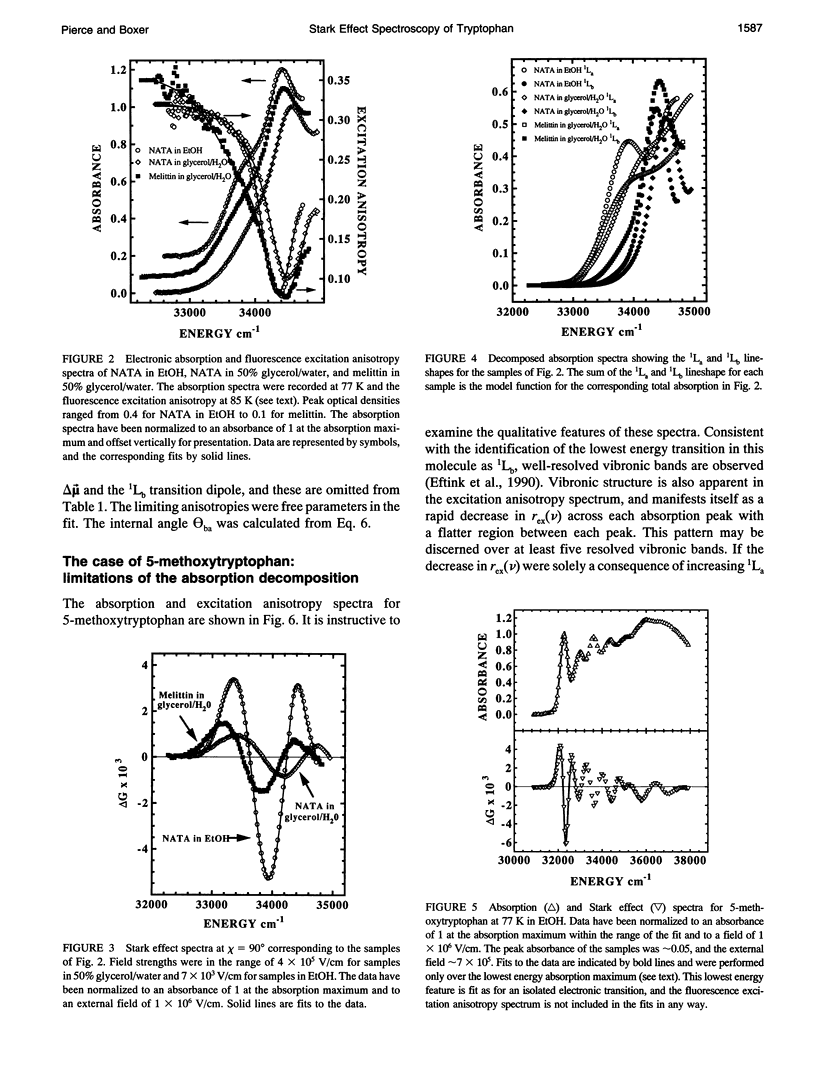
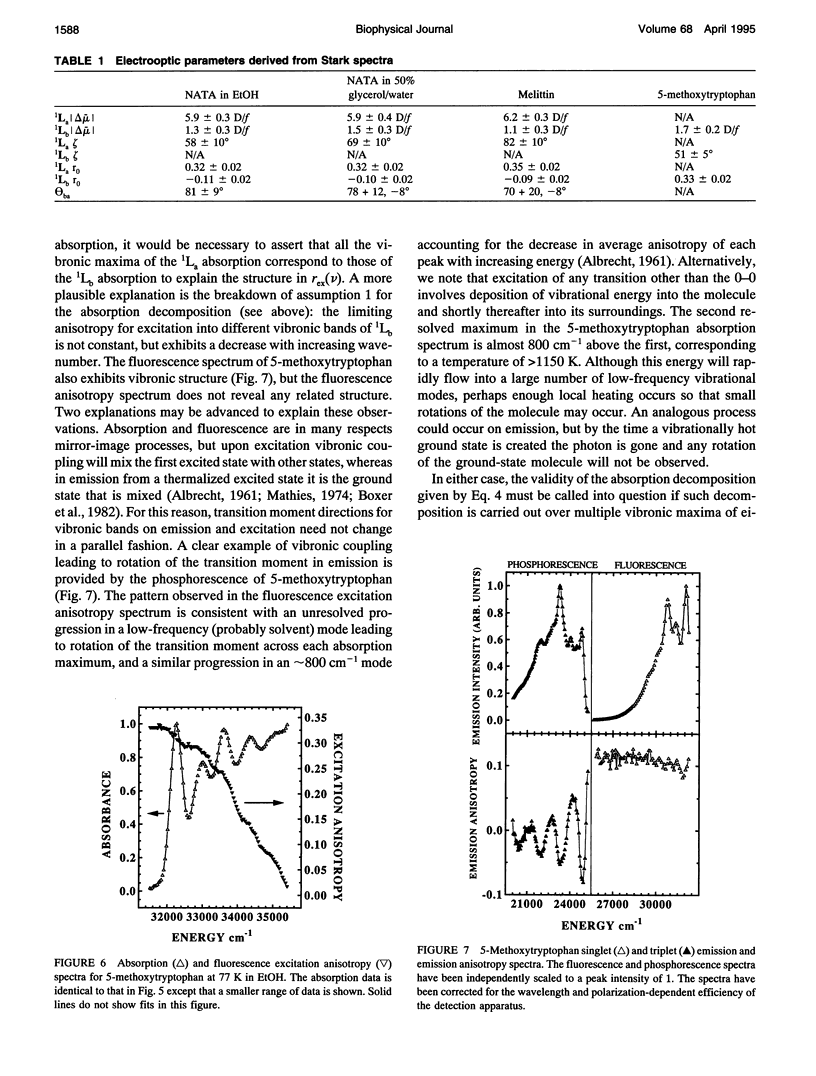
The change in permanent dipole moment (magnitude of delta mu) for the transition from the 1La state to the ground state of tryptophan is the key photophysical parameter for the interpretation of tryptophan fluorescence spectra in terms of static and dynamic dielectric properties of the surrounding medium. We report measurement of this parameter by means of electric field effect (Stark) spectroscopy for N-acetyl-L-tryptophanamide (NATA) in two solvents, the single tryptophan containing peptide melittin, and 5-methoxytryptophan. The values ranged from 5.9 to 6.2 +/- 0.4 Debye/f for NATA and melittin, where f represents the local field correction. The 1Lb magnitude of delta mu was much smaller. Application of Stark spectroscopy to these chromophores required decomposition of the near-UV absorption into the 1La and 1Lb bands by measurement of the fluorescence excitation anisotropy spectrum and represents an extension of the method to systems where band overlap would normally preclude quantitative analysis of the Stark spectrum. The results obtained for 5-methoxytryptophan point out limitations of this method of spectral decomposition. The relevance of these results to the interpretation of steady-state and time-resolved spectroscopy of tryptophan is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beechem J. M., Brand L. Time-resolved fluorescence of proteins. Annu Rev Biochem. 1985;54:43–71. doi: 10.1146/annurev.bi.54.070185.000355. [DOI] [PubMed] [Google Scholar]
- Boxer S. G., Kuki A., Wright K. A., Katz B. A., Xuong N. H. Oriented properties of the chlorophylls: Electronic absorption spectroscopy of orthorhombic pyrochlorophyllide a-apomyoglobin single crystals. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1121–1125. doi: 10.1073/pnas.79.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demchenko A. P., Ladokhin A. S. Red-edge-excitation fluorescence spectroscopy of indole and tryptophan. Eur Biophys J. 1988;15(6):369–379. doi: 10.1007/BF00254724. [DOI] [PubMed] [Google Scholar]
- Valeur B., Weber G. Resolution of the fluorescence excitation spectrum of indole into the 1La and 1Lb excitation bands. Photochem Photobiol. 1977 May;25(5):441–444. doi: 10.1111/j.1751-1097.1977.tb09168.x. [DOI] [PubMed] [Google Scholar]


