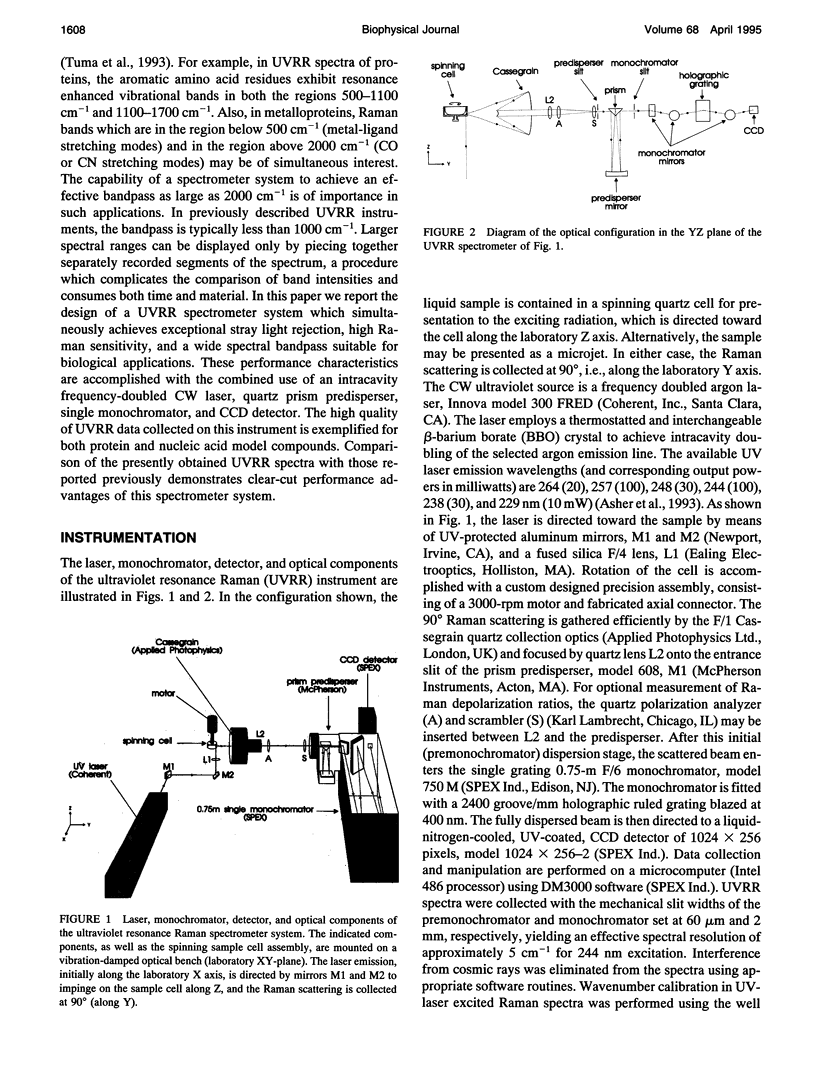
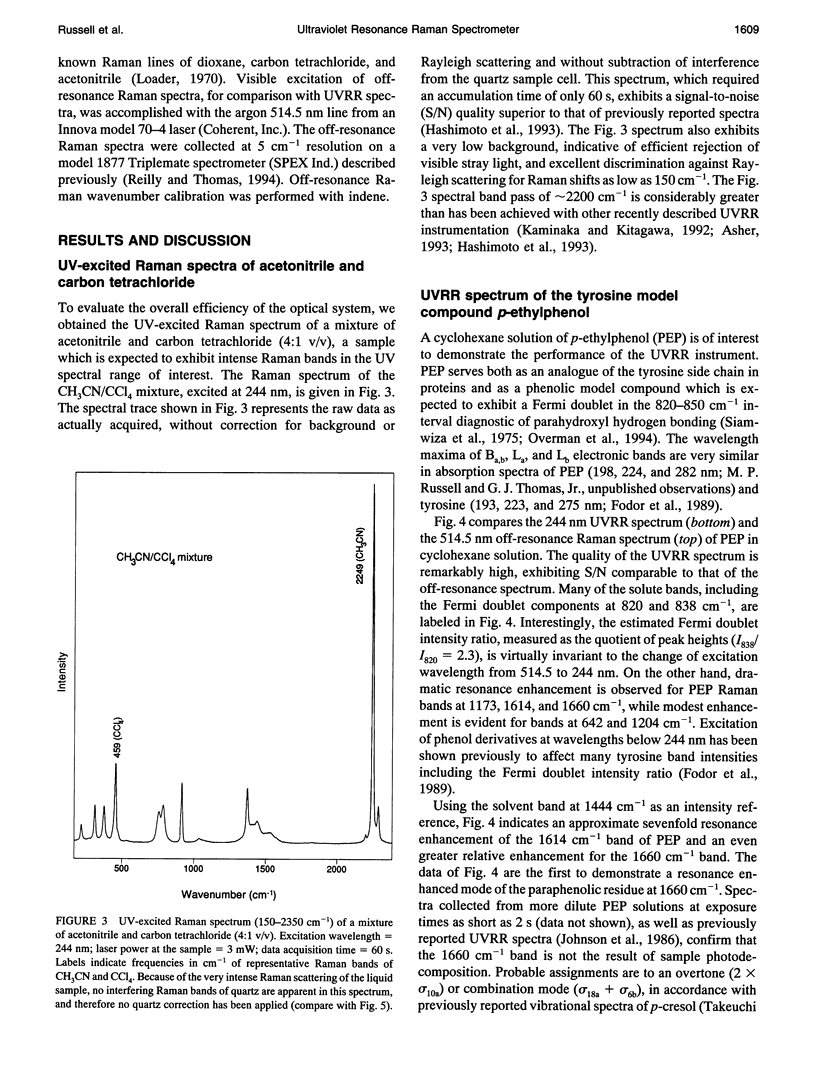
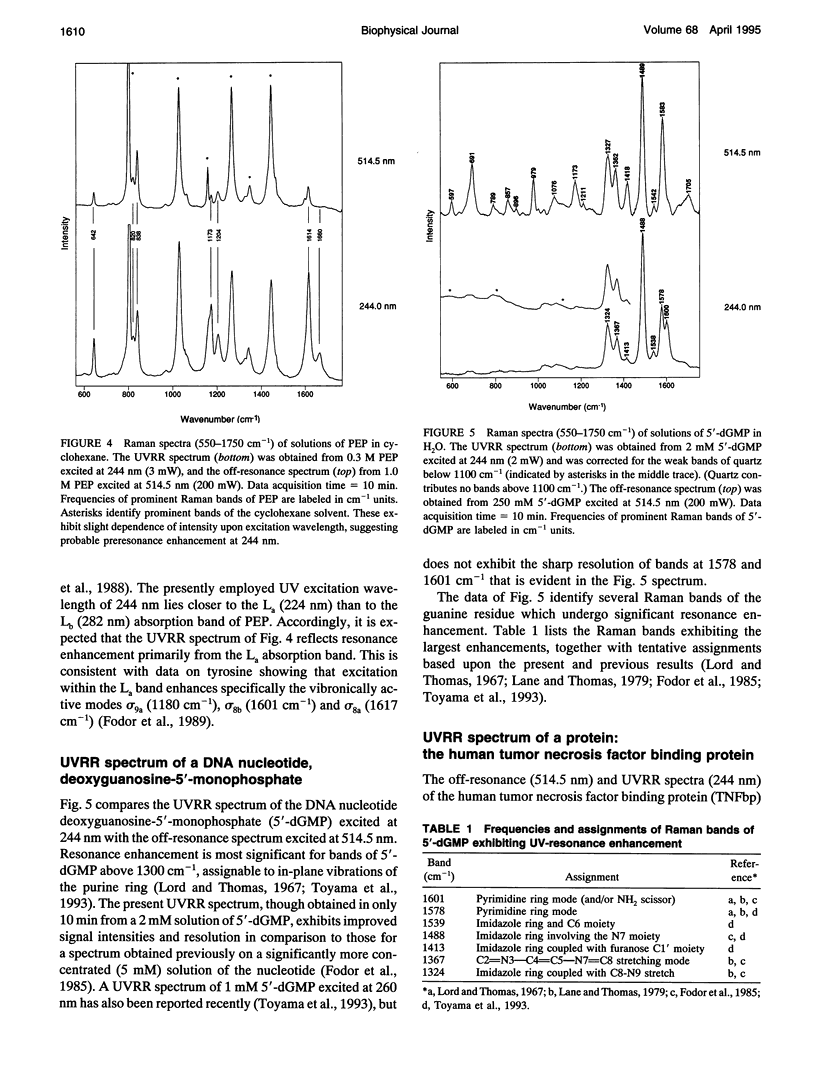
Abstract
We describe an ultraviolet resonance Raman (UVRR) spectrometer appropriate for structural studies of biological macromolecules and their assemblies. Instrument design includes the following features: a continuous wave, intracavity doubled, ultraviolet laser source for excitation of the Raman spectrum; a rotating cell (or jet source) for presentation of the sample to the laser beam; a Cassegrain optic with f/1.0 aperture for collection of the Raman scattering; a quartz prism dispersing element for rejection of stray light and Rayleigh scattering; a 0.75-m single grating monochromator for dispersion of the Raman scattering; and a liquid-nitrogen-cooled, charge-coupled device for detection of the Raman photons. The performance of this instrument, assessed on the basis of the observed signal-to-noise ratios, the apparent resolution of closely spaced spectral bands, and the wide spectrometer bandpass of 2200 cm-1, is believed superior to previously described UVRR spectrometers of similar design. Performance characteristics of the instrument are demonstrated in UVRR spectra obtained from standard solvents, p-ethylphenol, which serves as a model for the tyrosine side chain, the DNA nucleotide deoxyguanosine-5'-monophosphate, and the human tumor necrosis factor binding protein, which is considered representative of soluble globular proteins.
Full text
PDF
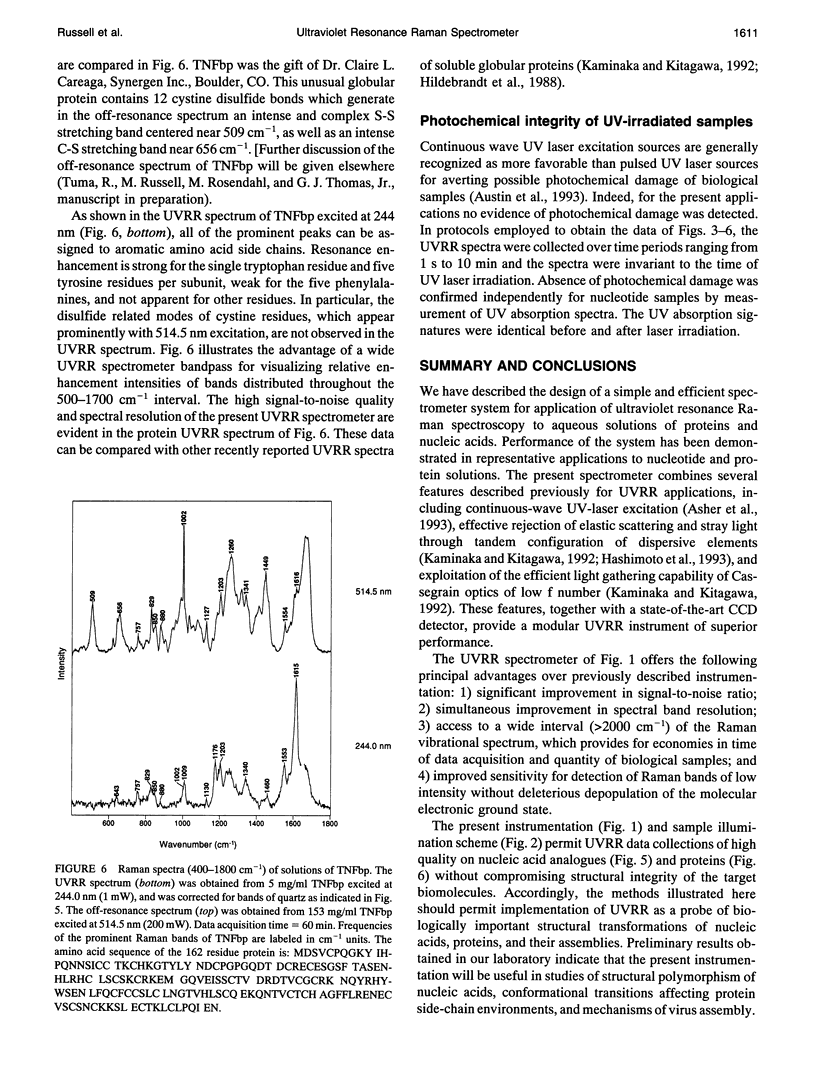

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hildebrandt P. G., Copeland R. A., Spiro T. G., Otlewski J., Laskowski M., Jr, Prendergast F. G. Tyrosine hydrogen-bonding and environmental effects in proteins probed by ultraviolet resonance Raman spectroscopy. Biochemistry. 1988 Jul 26;27(15):5426–5433. doi: 10.1021/bi00415a007. [DOI] [PubMed] [Google Scholar]
- Lane M. J., Thomas G. J., Jr Kinetics of hydrogen-deuterium exchange in guanosine 5'-monophosphate and guanosine 3':5'-monophosphate determined by laser-Raman spectroscopy. Biochemistry. 1979 Sep 4;18(18):3839–3846. doi: 10.1021/bi00585a002. [DOI] [PubMed] [Google Scholar]
- Overman S. A., Aubrey K. L., Vispo N. S., Cesareni G., Thomas G. J., Jr Novel tyrosine markers in Raman spectra of wild-type and mutant (Y21M and Y24M) Ff virions indicate unusual environments for coat protein phenoxyls. Biochemistry. 1994 Feb 8;33(5):1037–1042. doi: 10.1021/bi00171a001. [DOI] [PubMed] [Google Scholar]
- Reilly K. E., Thomas G. J., Jr Hydrogen exchange dynamics of the P22 virion determined by time-resolved Raman spectroscopy. Effects of chromosome packaging on the kinetics of nucleotide exchanges. J Mol Biol. 1994 Aug 5;241(1):68–82. doi: 10.1006/jmbi.1994.1474. [DOI] [PubMed] [Google Scholar]
- Siamwiza M. N., Lord R. C., Chen M. C., Takamatsu T., Harada I., Matsuura H., Shimanouchi T. Interpretation of the doublet at 850 and 830 cm-1 in the Raman spectra of tyrosyl residues in proteins and certain model compounds. Biochemistry. 1975 Nov 4;14(22):4870–4876. doi: 10.1021/bi00693a014. [DOI] [PubMed] [Google Scholar]
- Tuma R., Vohník S., Li H., Thomas G. J., Jr Cysteine conformation and sulfhydryl interactions in proteins and viruses. 3. Quantitative measurement of the Raman S-H band intensity and frequency. Biophys J. 1993 Sep;65(3):1066–1072. doi: 10.1016/S0006-3495(93)81172-X. [DOI] [PMC free article] [PubMed] [Google Scholar]