Abstract
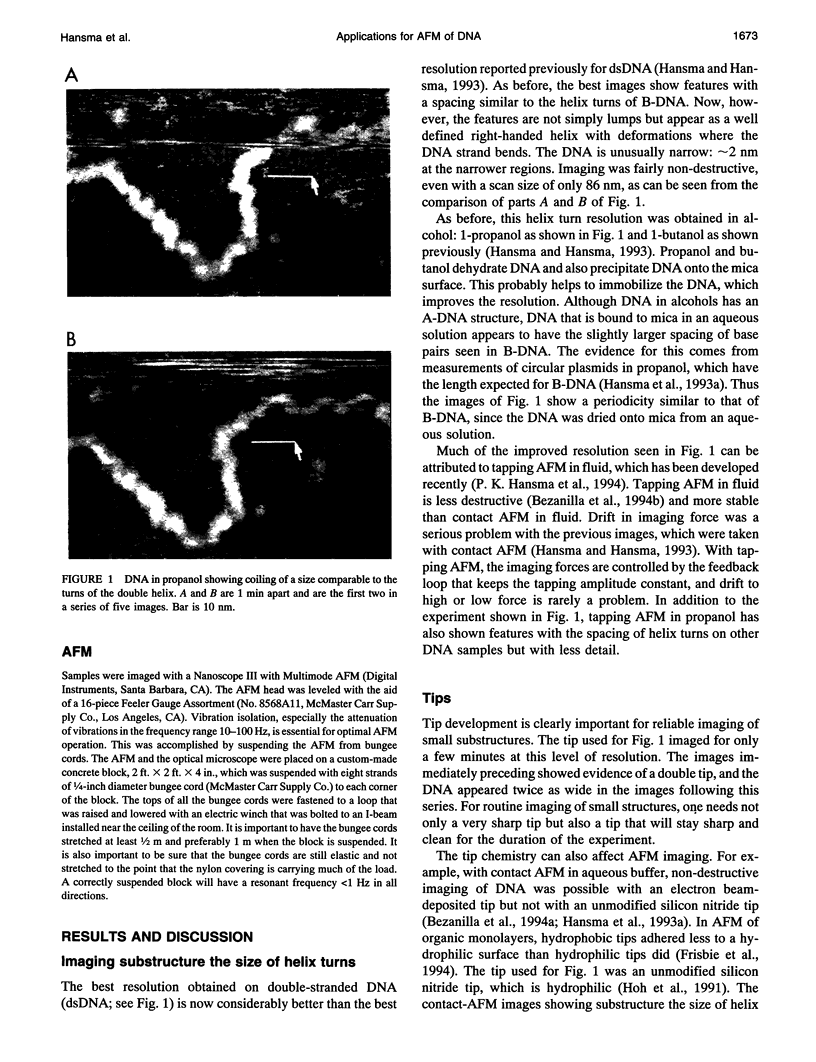
Tapping mode atomic force microscopy (AFM) of DNA in propanol, dry helium, and aqueous buffer each have specific applications. Resolution is best in propanol, which precipitates and immobilizes the DNA and provides a fluid imaging environment where adhesive forces are minimized. Resolution on exceptional images of DNA appears to be approximately 2 nm, sufficient to see helix turns in detail, but the smallest substructures typically seen on DNA in propanol are approximately 6-10 nm in size. Tapping AFM in dry helium provides a convenient way of imaging such things as conformations of DNA molecules and positions of proteins on DNA. Images of single-stranded DNA and RecA-DNA complexes are presented. In aqueous buffer DNA molecules as small as 300 bp have been imaged even when in motion. Images are presented of the changes in shape and position of circular plasmid DNA molecules.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. J., Dong X. F., O'Neill T. E., Yau P., Kowalczykowski S. C., Gatewood J., Balhorn R., Bradbury E. M. Atomic force microscope measurements of nucleosome cores assembled along defined DNA sequences. Biochemistry. 1993 Aug 24;32(33):8390–8396. doi: 10.1021/bi00084a002. [DOI] [PubMed] [Google Scholar]
- Bezanilla M., Drake B., Nudler E., Kashlev M., Hansma P. K., Hansma H. G. Motion and enzymatic degradation of DNA in the atomic force microscope. Biophys J. 1994 Dec;67(6):2454–2459. doi: 10.1016/S0006-3495(94)80733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante C., Vesenka J., Tang C. L., Rees W., Guthold M., Keller R. Circular DNA molecules imaged in air by scanning force microscopy. Biochemistry. 1992 Jan 14;31(1):22–26. doi: 10.1021/bi00116a005. [DOI] [PubMed] [Google Scholar]
- Frisbie C. D., Rozsnyai L. F., Noy A., Wrighton M. S., Lieber C. M. Functional group imaging by chemical force microscopy. Science. 1994 Sep 30;265(5181):2071–2074. doi: 10.1126/science.265.5181.2071. [DOI] [PubMed] [Google Scholar]
- Guthold M., Bezanilla M., Erie D. A., Jenkins B., Hansma H. G., Bustamante C. Following the assembly of RNA polymerase-DNA complexes in aqueous solutions with the scanning force microscope. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12927–12931. doi: 10.1073/pnas.91.26.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman P. J. Flexibility of DNA. Annu Rev Biophys Biophys Chem. 1988;17:265–286. doi: 10.1146/annurev.bb.17.060188.001405. [DOI] [PubMed] [Google Scholar]
- Hansma H. G., Bezanilla M., Zenhausern F., Adrian M., Sinsheimer R. L. Atomic force microscopy of DNA in aqueous solutions. Nucleic Acids Res. 1993 Feb 11;21(3):505–512. doi: 10.1093/nar/21.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansma H. G., Browne K. A., Bezanilla M., Bruice T. C. Bending and straightening of DNA induced by the same ligand: characterization with the atomic force microscope. Biochemistry. 1994 Jul 19;33(28):8436–8441. doi: 10.1021/bi00194a007. [DOI] [PubMed] [Google Scholar]
- Hansma H. G., Hoh J. H. Biomolecular imaging with the atomic force microscope. Annu Rev Biophys Biomol Struct. 1994;23:115–139. doi: 10.1146/annurev.bb.23.060194.000555. [DOI] [PubMed] [Google Scholar]
- Hansma H. G., Sinsheimer R. L., Groppe J., Bruice T. C., Elings V., Gurley G., Bezanilla M., Mastrangelo I. A., Hough P. V., Hansma P. K. Recent advances in atomic force microscopy of DNA. Scanning. 1993 Sep-Oct;15(5):296–299. doi: 10.1002/sca.4950150509. [DOI] [PubMed] [Google Scholar]
- Hansma H. G., Vesenka J., Siegerist C., Kelderman G., Morrett H., Sinsheimer R. L., Elings V., Bustamante C., Hansma P. K. Reproducible imaging and dissection of plasmid DNA under liquid with the atomic force microscope. Science. 1992 May 22;256(5060):1180–1184. doi: 10.1126/science.256.5060.1180. [DOI] [PubMed] [Google Scholar]
- Lyubchenko Y., Shlyakhtenko L., Harrington R., Oden P., Lindsay S. Atomic force microscopy of long DNA: imaging in air and under water. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2137–2140. doi: 10.1073/pnas.90.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menetski J. P., Bear D. G., Kowalczykowski S. C. Stable DNA heteroduplex formation catalyzed by the Escherichia coli RecA protein in the absence of ATP hydrolysis. Proc Natl Acad Sci U S A. 1990 Jan;87(1):21–25. doi: 10.1073/pnas.87.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. N., Hansma H. G., Bezanilla M., Sano T., Ogletree D. F., Kolbe W., Smith C. L., Cantor C. R., Spengler S., Hansma P. K. Atomic force microscopy of biochemically tagged DNA. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3811–3814. doi: 10.1073/pnas.90.9.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees W. A., Keller R. W., Vesenka J. P., Yang G., Bustamante C. Evidence of DNA bending in transcription complexes imaged by scanning force microscopy. Science. 1993 Jun 11;260(5114):1646–1649. doi: 10.1126/science.8503010. [DOI] [PubMed] [Google Scholar]
- Samorí B., Siligardi G., Quagliariello C., Weisenhorn A. L., Vesenka J., Bustamante C. J. Chirality of DNA supercoiling assigned by scanning force microscopy. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3598–3601. doi: 10.1073/pnas.90.8.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DK, Steinberg S, Israelachvili J, Zasadzinski JA. Growth of a self-assembled monolayer by fractal aggregation. Phys Rev Lett. 1992 Dec 7;69(23):3354–3357. doi: 10.1103/PhysRevLett.69.3354. [DOI] [PubMed] [Google Scholar]
- Shaiu W. L., Larson D. D., Vesenka J., Henderson E. Atomic force microscopy of oriented linear DNA molecules labeled with 5nm gold spheres. Nucleic Acids Res. 1993 Jan 11;21(1):99–103. doi: 10.1093/nar/21.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thundat T., Allison D. P., Warmack R. J. Stretched DNA structures observed with atomic force microscopy. Nucleic Acids Res. 1994 Oct 11;22(20):4224–4228. doi: 10.1093/nar/22.20.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesenka J., Guthold M., Tang C. L., Keller D., Delaine E., Bustamante C. Substrate preparation for reliable imaging of DNA molecules with the scanning force microscope. Ultramicroscopy. 1992 Jul;42-44(Pt B):1243–1249. doi: 10.1016/0304-3991(92)90430-r. [DOI] [PubMed] [Google Scholar]
- Weisenhorn A. L., Gaub H. E., Hansma H. G., Sinsheimer R. L., Kelderman G. L., Hansma P. K. Imaging single-stranded DNA, antigen-antibody reaction and polymerized Langmuir-Blodgett films with an atomic force microscope. Scanning Microsc. 1990 Sep;4(3):511–516. [PubMed] [Google Scholar]
- Weisenhorn AL, Maivald P, Butt H, Hansma PK. Measuring adhesion, attraction, and repulsion between surfaces in liquids with an atomic-force microscope. Phys Rev B Condens Matter. 1992 May 15;45(19):11226–11232. doi: 10.1103/physrevb.45.11226. [DOI] [PubMed] [Google Scholar]
- West S. C. Enzymes and molecular mechanisms of genetic recombination. Annu Rev Biochem. 1992;61:603–640. doi: 10.1146/annurev.bi.61.070192.003131. [DOI] [PubMed] [Google Scholar]