Abstract
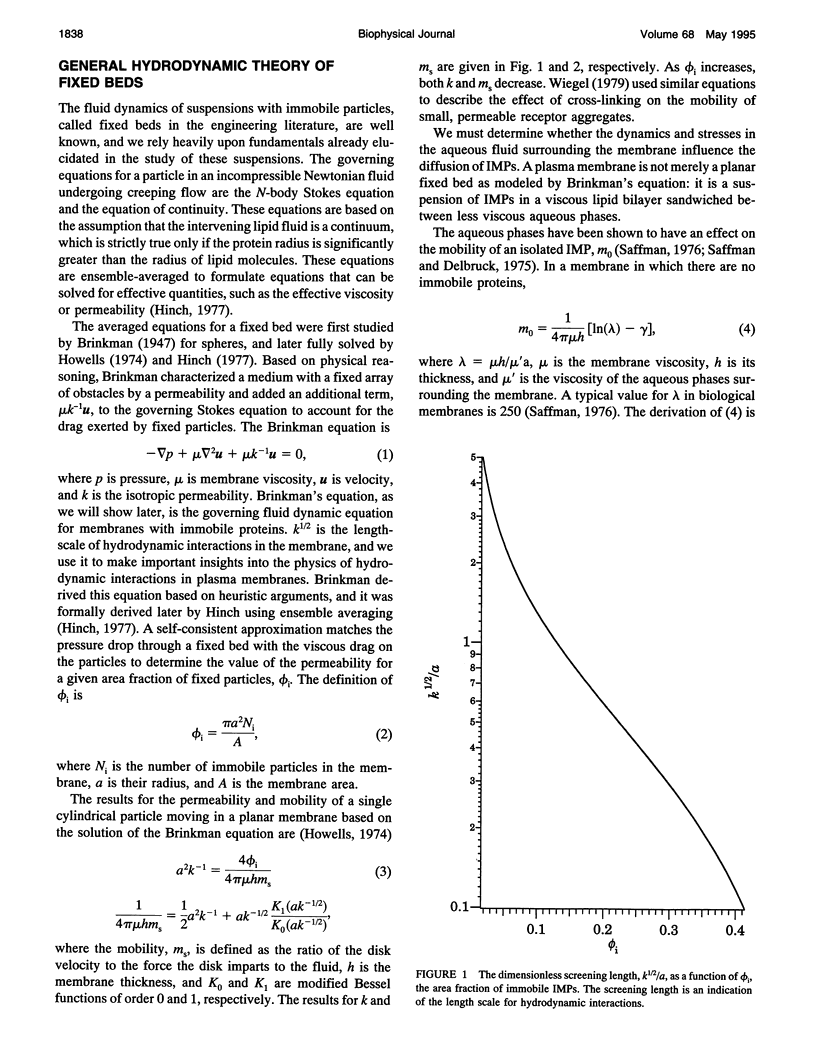
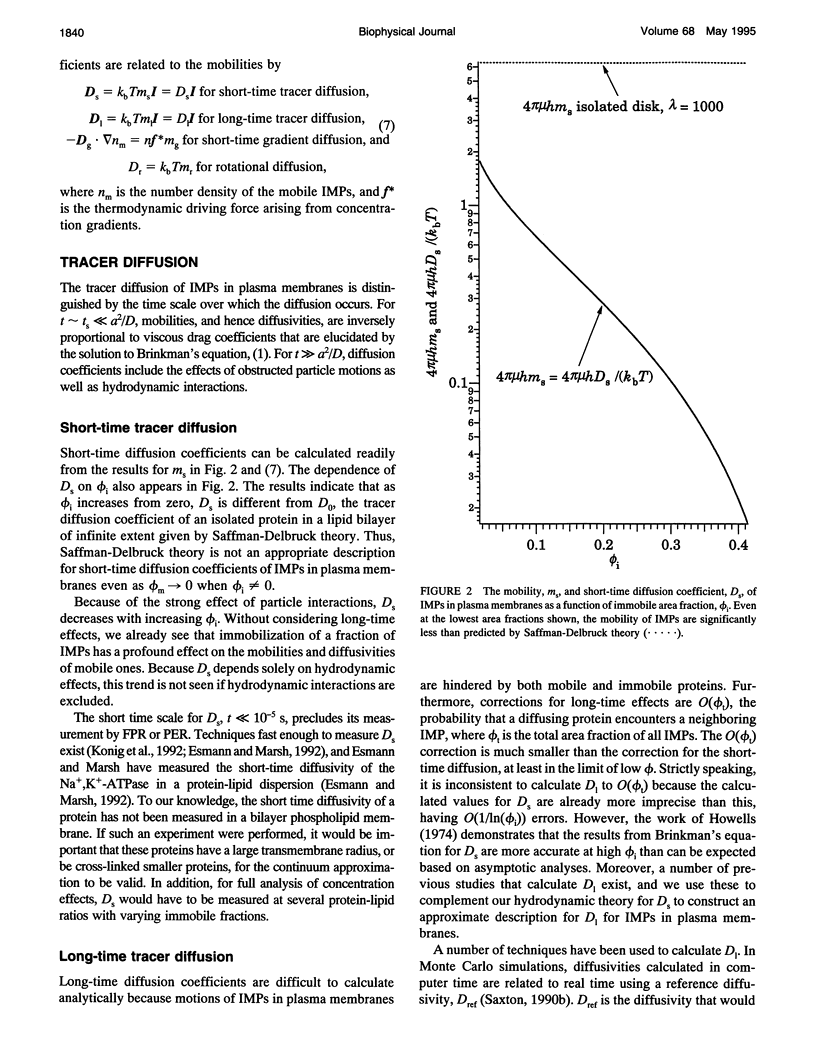
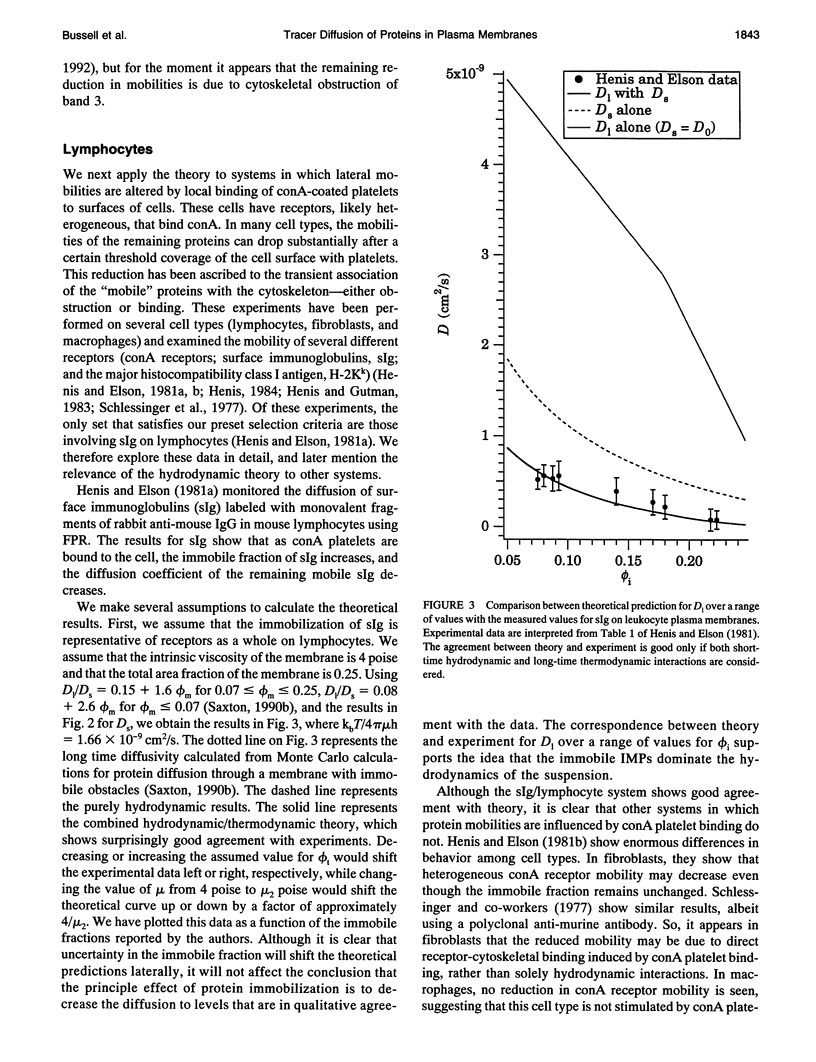
Tracer diffusion coefficients of integral membrane proteins (IMPs) in intact plasma membranes are often much lower than those found in blebbed, organelle, and reconstituted membranes. We calculate the contribution of hydrodynamic interactions to the tracer, gradient, and rotational diffusion of IMPs in plasma membranes. Because of the presence of immobile IMPs, Brinkman's equation governs the hydrodynamics in plasma membranes. Solutions of Brinkman's equation enable the calculation of short-time diffusion coefficients of IMPs. There is a large reduction in particle mobilities when a fraction of them is immobile, and as the fraction increases, the mobilities of the mobile particles continue to decrease. Combination of the hydrodynamic mobilities with Monte Carlo simulation results, which incorporate excluded area effects, enable the calculation of long-time diffusion coefficients. We use our calculations to analyze results for tracer diffusivities in several different systems. In erythrocytes, we find that the hydrodynamic theory, when combined with excluded area effects, closes the gap between existing theory and experiment for the mobility of band 3, with the remaining discrepancy likely due to direct obstruction of band 3 lateral mobility by the spectrin network. In lymphocytes, the combined hydrodynamic-excluded area theory provides a plausible explanation for the reduced mobility of sIg molecules induced by binding concanavalin A-coated platelets. However, the theory does not explain all reported cases of "anchorage modulation" in all cell types in which receptor mobilities are reduced after binding by concanavalin A-coated platelets. The hydrodynamic theory provides an explanation of why protein lateral mobilities are restricted in plasma membranes and why, in many systems, deletion of the cytoplasmic tail of a receptor has little effect on diffusion rates. However, much more data are needed to test the theory definitively. We also predict that gradient and tracer diffusivities are the same to leading order. Finally, we have calculated rotational diffusion coefficients in plasma membranes. They decrease less rapidly than translational diffusion coefficients with increasing protein immobilization, and the results agree qualitatively with the limited experimental data available.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney J. R., Scalettar B. A., Owicki J. C. Mutual diffusion of interacting membrane proteins. Biophys J. 1989 Aug;56(2):315–326. doi: 10.1016/S0006-3495(89)82678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agre P., Preston G. M., Smith B. L., Jung J. S., Raina S., Moon C., Guggino W. B., Nielsen S. Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol. 1993 Oct;265(4 Pt 2):F463–F476. doi: 10.1152/ajprenal.1993.265.4.F463. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V. The spectrin-actin junction of erythrocyte membrane skeletons. Biochim Biophys Acta. 1989 Jan 18;988(1):107–121. doi: 10.1016/0304-4157(89)90006-3. [DOI] [PubMed] [Google Scholar]
- Bierer B. E., Herrmann S. H., Brown C. S., Burakoff S. J., Golan D. E. Lateral mobility of class I histocompatibility antigens in B lymphoblastoid cell membranes: modulation by cross-linking and effect of cell density. J Cell Biol. 1987 Sep;105(3):1147–1152. doi: 10.1083/jcb.105.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussell S. J., Koch D. L., Hammer D. A. Effect of hydrodynamic interactions on the diffusion of integral membrane proteins: tracer diffusion in organelle and reconstituted membranes. Biophys J. 1995 May;68(5):1828–1835. doi: 10.1016/S0006-3495(95)80359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett J. D., Agre P., Palek J., Golan D. E. Differential control of band 3 lateral and rotational mobility in intact red cells. J Clin Invest. 1994 Aug;94(2):683–688. doi: 10.1172/JCI117385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanovich S., Trón L., Szöllösi J., Zidovetzki R., Vaz W. L., Regateiro F., Arndt-Jovin D. J., Jovin T. M. Distribution and mobility of murine histocompatibility H-2Kk antigen in the cytoplasmic membrane. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5985–5989. doi: 10.1073/pnas.80.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M., Stroynowski I. Differences between the lateral organization of conventional and inositol phospholipid-anchored membrane proteins. A further definition of micrometer scale membrane domains. J Cell Biol. 1991 Mar;112(6):1143–1150. doi: 10.1083/jcb.112.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M., Zuniga M. Lateral diffusion of wild-type and mutant Ld antigens in L cells. J Cell Biol. 1984 Dec;99(6):2333–2335. doi: 10.1083/jcb.99.6.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. W., Posner R. G., Goldstein B., Holowka D., Baird B. Bivalent ligand dissociation kinetics from receptor-bound immunoglobulin E: evidence for a time-dependent increase in ligand rebinding at the cell surface. Biochemistry. 1991 Mar 5;30(9):2357–2363. doi: 10.1021/bi00223a009. [DOI] [PubMed] [Google Scholar]
- Esmann M., Marsh D. Local translational diffusion rates of membranous Na+,K(+)-ATPase measured by saturation transfer ESR spectroscopy. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7606–7609. doi: 10.1073/pnas.89.16.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan D. E., Alecio M. R., Veatch W. R., Rando R. R. Lateral mobility of phospholipid and cholesterol in the human erythrocyte membrane: effects of protein-lipid interactions. Biochemistry. 1984 Jan 17;23(2):332–339. doi: 10.1021/bi00297a024. [DOI] [PubMed] [Google Scholar]
- Goldstein B., Wofsy C., Bell G. Interactions of low density lipoprotein receptors with coated pits on human fibroblasts: estimate of the forward rate constant and comparison with the diffusion limit. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5695–5698. doi: 10.1073/pnas.78.9.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henis Y. I., Elson E. L. Differences in the response of several cell types to inhibition of surface receptor mobility by local concanavalin A binding. Exp Cell Res. 1981 Nov;136(1):189–201. doi: 10.1016/0014-4827(81)90050-1. [DOI] [PubMed] [Google Scholar]
- Henis Y. I., Elson E. L. Inhibition of the mobility of mouse lymphocyte surface immunoglobulins by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1072–1076. doi: 10.1073/pnas.78.2.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henis Y. I., Gutman O. Lateral diffusion and patch formation of H-2Kk antigens on mouse spleen lymphocytes. Biochim Biophys Acta. 1983 Apr 5;762(2):281–288. doi: 10.1016/0167-4889(83)90082-4. [DOI] [PubMed] [Google Scholar]
- Henis Y. I. Mobility modulation by local concanavalin A binding. Selectivity toward different membrane proteins. J Biol Chem. 1984 Feb 10;259(3):1515–1519. [PubMed] [Google Scholar]
- Livneh E., Benveniste M., Prywes R., Felder S., Kam Z., Schlessinger J. Large deletions in the cytoplasmic kinase domain of the epidermal growth factor receptor do not affect its laternal mobility. J Cell Biol. 1986 Aug;103(2):327–331. doi: 10.1083/jcb.103.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey M. A., Liu Z. Y., Poo M. M. Lateral electromigration and diffusion of Fc epsilon receptors on rat basophilic leukemia cells: effects of IgE binding. J Cell Biol. 1984 Sep;99(3):778–787. doi: 10.1083/jcb.99.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Noyola M. Long-time self-diffusion in concentrated colloidal dispersions. Phys Rev Lett. 1988 Jun 27;60(26):2705–2708. doi: 10.1103/PhysRevLett.60.2705. [DOI] [PubMed] [Google Scholar]
- Metzger H., Alcaraz G., Hohman R., Kinet J. P., Pribluda V., Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- Myers J. N., Holowka D., Baird B. Rotational motion of monomeric and dimeric immunoglobulin E-receptor complexes. Biochemistry. 1992 Jan 21;31(2):567–575. doi: 10.1021/bi00117a038. [DOI] [PubMed] [Google Scholar]
- Peters R., Cherry R. J. Lateral and rotational diffusion of bacteriorhodopsin in lipid bilayers: experimental test of the Saffman-Delbrück equations. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4317–4321. doi: 10.1073/pnas.79.14.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M. In situ electrophoresis of membrane components. Annu Rev Biophys Bioeng. 1981;10:245–276. doi: 10.1146/annurev.bb.10.060181.001333. [DOI] [PubMed] [Google Scholar]
- Pownall H. J., Massey J. B. Mechanism of association of human plasma apolipoproteins with dimyristoylphosphatidylcholine: effect of lipid clusters on reaction rates. Biophys J. 1982 Jan;37(1):177–179. doi: 10.1016/S0006-3495(82)84659-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman N. A., Pecht I., Roess D. A., Barisas B. G. Rotational dynamics of type I Fc epsilon receptors on individually-selected rat mast cells studied by polarized fluorescence depletion. Biophys J. 1992 Feb;61(2):334–346. doi: 10.1016/S0006-3495(92)81840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in a mixture of mobile and immobile particles. A Monte Carlo study. Biophys J. 1990 Nov;58(5):1303–1306. doi: 10.1016/S0006-3495(90)82470-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. The membrane skeleton of erythrocytes. A percolation model. Biophys J. 1990 Jun;57(6):1167–1177. doi: 10.1016/S0006-3495(90)82636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Elson E. L., Webb W. W., Yahara I., Rutishauser U., Edelman G. M. Receptor diffusion on cell surfaces modulated by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1110–1114. doi: 10.1073/pnas.74.3.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullion B. F., Hou Y., Puddington L., Rose J. K., Jacobson K. Effects of mutations in three domains of the vesicular stomatitis viral glycoprotein on its lateral diffusion in the plasma membrane. J Cell Biol. 1987 Jul;105(1):69–75. doi: 10.1083/jcb.105.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Schindler M., Koppel D. E. Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature. 1980 Jun 12;285(5765):510–511. doi: 10.1038/285510a0. [DOI] [PubMed] [Google Scholar]
- Tank D. W., Wu E. S., Webb W. W. Enhanced molecular diffusibility in muscle membrane blebs: release of lateral constraints. J Cell Biol. 1982 Jan;92(1):207–212. doi: 10.1083/jcb.92.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. L., Feder T. J., Webb W. W. Effects of protein concentration on IgE receptor mobility in rat basophilic leukemia cell plasma membranes. Biophys J. 1992 May;61(5):1402–1412. doi: 10.1016/S0006-3495(92)81946-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji A., Ohnishi S. Restriction of the lateral motion of band 3 in the erythrocyte membrane by the cytoskeletal network: dependence on spectrin association state. Biochemistry. 1986 Oct 7;25(20):6133–6139. doi: 10.1021/bi00368a045. [DOI] [PubMed] [Google Scholar]
- Wade W. F., Freed J. H., Edidin M. Translational diffusion of class II major histocompatibility complex molecules is constrained by their cytoplasmic domains. J Cell Biol. 1989 Dec;109(6 Pt 2):3325–3331. doi: 10.1083/jcb.109.6.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegel F. W. Hydrodynamics of a permeable patch in the fluid membrane. J Theor Biol. 1979 Mar 21;77(2):189–193. doi: 10.1016/0022-5193(79)90306-0. [DOI] [PubMed] [Google Scholar]
- Zagyansky Y. A., Jard S. Does lectin-receptor complex formation produce zones of restricted mobility within the membrane? Nature. 1979 Aug 16;280(5723):591–593. doi: 10.1038/280591a0. [DOI] [PubMed] [Google Scholar]
- Zhang F., Lee G. M., Jacobson K. Protein lateral mobility as a reflection of membrane microstructure. Bioessays. 1993 Sep;15(9):579–588. doi: 10.1002/bies.950150903. [DOI] [PubMed] [Google Scholar]
- Zidovetzki R., Bartholdi M., Arndt-Jovin D., Jovin T. M. Rotational dynamics of the Fc receptor for immunoglobulin E on histamine-releasing rat basophilic leukemia cells. Biochemistry. 1986 Jul 29;25(15):4397–4401. doi: 10.1021/bi00363a033. [DOI] [PubMed] [Google Scholar]


