Abstract
The effect of antibodies to glycophorin A on the rotational diffusion of band 3 in human erythrocyte membranes was investigated by transient dichrosim. Three antibodies that recognize different epitopes on the exofacial domain of glycophorin A all strongly reduce the rotational mobility of band 3. The effect is at most only weakly dependent on the distance of the epitope from the membrane surface. The degree of immobilization obtained with two of the antibodies, BRIC14 and R18, is very similar to that produced by antibodies to band 3 itself. Similar results were obtained with membranes stripped of skeletal proteins. Fab fragments and an antibody to glycophorin C had no effect on band 3 rotational mobility. These results rule out a mechanism whereby band 3 rotational immobilization results from enhanced interactions with the membrane skeleton that are mediated by a conformational change in glycophorin A. Rather, they strongly indicate that the antibodies to glycophorin A cross-link existing band 3-glycophorin A complexes that have lifetimes that are long compared with the millisecond time scale of the transient dichroism measurements.
Full text
PDF

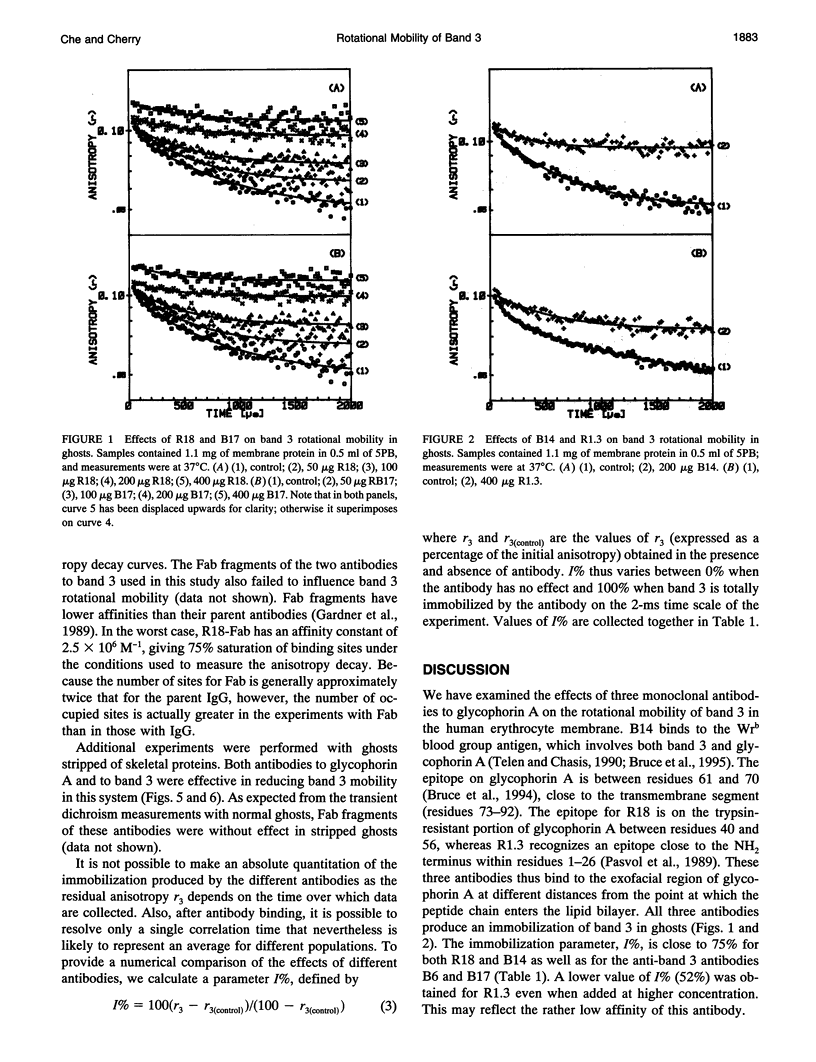
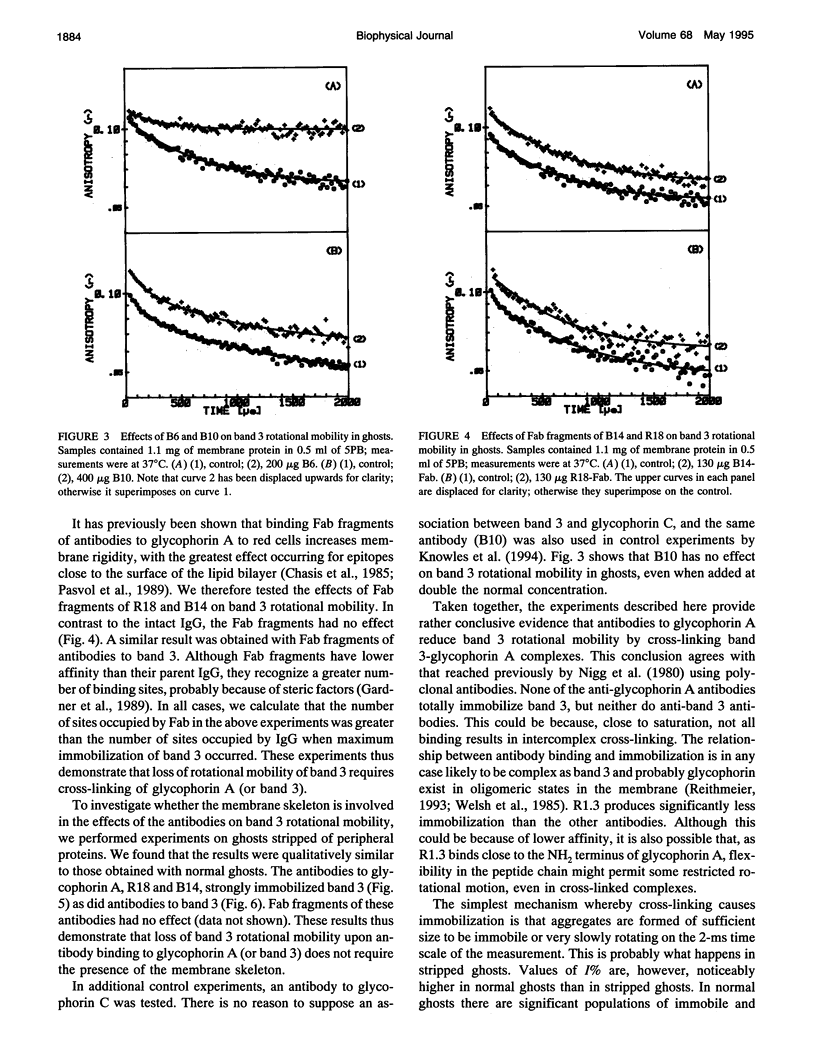



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce L. J., Ring S. M., Anstee D. J., Reid M. E., Wilkinson S., Tanner M. J. Changes in the blood group Wright antigens are associated with a mutation at amino acid 658 in human erythrocyte band 3: a site of interaction between band 3 and glycophorin A under certain conditions. Blood. 1995 Jan 15;85(2):541–547. [PubMed] [Google Scholar]
- Chasis J. A., Mohandas N., Shohet S. B. Erythrocyte membrane rigidity induced by glycophorin A-ligand interaction. Evidence for a ligand-induced association between glycophorin A and skeletal proteins. J Clin Invest. 1985 Jun;75(6):1919–1926. doi: 10.1172/JCI111907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasis J. A., Reid M. E., Jensen R. H., Mohandas N. Signal transduction by glycophorin A: role of extracellular and cytoplasmic domains in a modulatable process. J Cell Biol. 1988 Oct;107(4):1351–1357. doi: 10.1083/jcb.107.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che A., Cherry R. J., Bannister L. H., Dluzewski A. R. Aggregation of band 3 in hereditary ovalocytic red blood cell membranes. Electron microscopy and protein rotational diffusion studies. J Cell Sci. 1993 Jul;105(Pt 3):655–660. doi: 10.1242/jcs.105.3.655. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Cogoli A., Oppliger M., Schneider G., Semenza G. A spectroscopic technique for measuring slow rotational diffusion of macromolecules. 1: Preparation and properties of a triplet probe. Biochemistry. 1976 Aug 24;15(17):3653–3656. doi: 10.1021/bi00662a001. [DOI] [PubMed] [Google Scholar]
- Cherry R. J. Measurement of protein rotational diffusion in membranes by flash photolysis. Methods Enzymol. 1978;54:47–61. doi: 10.1016/s0076-6879(78)54007-x. [DOI] [PubMed] [Google Scholar]
- Clague M. J., Harrison J. P., Cherry R. J. Cytoskeletal restraints of band 3 rotational mobility in human erythrocyte membranes. Biochim Biophys Acta. 1989 May 19;981(1):43–50. doi: 10.1016/0005-2736(89)90080-1. [DOI] [PubMed] [Google Scholar]
- Clough B., Paulitschke M., Nash G. B., Bayley P. M., Anstee D. J., Wilson R. J., Pasvol G., Gratzer W. B. Mechanism of regulation of malarial invasion by extraerythrocytic ligands. Mol Biochem Parasitol. 1995 Jan;69(1):19–27. doi: 10.1016/0166-6851(94)00185-p. [DOI] [PubMed] [Google Scholar]
- Gardner B., Parsons S. F., Merry A. H., Anstee D. J. Epitopes on sialoglycoprotein alpha: evidence for heterogeneity in the molecule. Immunology. 1989 Oct;68(2):283–289. [PMC free article] [PubMed] [Google Scholar]
- Groves J. D., Tanner M. J. Glycophorin A facilitates the expression of human band 3-mediated anion transport in Xenopus oocytes. J Biol Chem. 1992 Nov 5;267(31):22163–22170. [PubMed] [Google Scholar]
- Hadley T. J., Erkmen Z., Kaufman B. M., Futrovsky S., McGuinnis M. H., Graves P., Sadoff J. C., Miller L. H. Factors influencing invasion of erythrocytes by Plasmodium falciparum parasites: the effects of an N-acetyl glucosamine neoglycoprotein and an anti-glycophorin A antibody. Am J Trop Med Hyg. 1986 Sep;35(5):898–905. doi: 10.4269/ajtmh.1986.35.898. [DOI] [PubMed] [Google Scholar]
- Jarolim P., Rubin H. L., Liu S. C., Cho M. R., Brabec V., Derick L. H., Yi S. J., Saad S. T., Alper S., Brugnara C. Duplication of 10 nucleotides in the erythroid band 3 (AE1) gene in a kindred with hereditary spherocytosis and band 3 protein deficiency (band 3PRAGUE). J Clin Invest. 1994 Jan;93(1):121–130. doi: 10.1172/JCI116935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles D. W., Chasis J. A., Evans E. A., Mohandas N. Cooperative action between band 3 and glycophorin A in human erythrocytes: immobilization of band 3 induced by antibodies to glycophorin A. Biophys J. 1994 May;66(5):1726–1732. doi: 10.1016/S0006-3495(94)80965-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matayoshi E. D., Jovin T. M. Rotational diffusion of band 3 in erythrocyte membranes. 1. Comparison of ghosts and intact cells. Biochemistry. 1991 Apr 9;30(14):3527–3538. doi: 10.1021/bi00228a025. [DOI] [PubMed] [Google Scholar]
- McPherson R. A., Sawyer W. H., Tilley L. Band 3 mobility in camelid elliptocytes: implications for erythrocyte shape. Biochemistry. 1993 Jul 6;32(26):6696–6702. doi: 10.1021/bi00077a024. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Winardi R., Knowles D., Leung A., Parra M., George E., Conboy J., Chasis J. Molecular basis for membrane rigidity of hereditary ovalocytosis. A novel mechanism involving the cytoplasmic domain of band 3. J Clin Invest. 1992 Feb;89(2):686–692. doi: 10.1172/JCI115636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Bron C., Girardet M., Cherry R. J. Band 3-glycophorin A association in erythrocyte membrane demonstrated by combining protein diffusion measurements with antibody-induced cross-linking. Biochemistry. 1980 Apr 29;19(9):1887–1893. doi: 10.1021/bi00550a024. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Cherry R. J. Anchorage of a band 3 population at the erythrocyte cytoplasmic membrane surface: protein rotational diffusion measurements. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4702–4706. doi: 10.1073/pnas.77.8.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Cherry R. J. Influence of temperature and cholesterol on the rotational diffusion of band 3 in the human erythrocyte membrane. Biochemistry. 1979 Aug 7;18(16):3457–3465. doi: 10.1021/bi00583a004. [DOI] [PubMed] [Google Scholar]
- Pasvol G., Chasis J. A., Mohandas N., Anstee D. J., Tanner M. J., Merry A. H. Inhibition of malarial parasite invasion by monoclonal antibodies against glycophorin A correlates with reduction in red cell membrane deformability. Blood. 1989 Oct;74(5):1836–1843. [PubMed] [Google Scholar]
- Pinder J. C., Pekrun A., Maggs A. M., Gratzer W. B. Interaction of the red cell membrane skeleton with the membrane. Biochem Soc Trans. 1992 Nov;20(4):774–776. doi: 10.1042/bst0200774. [DOI] [PubMed] [Google Scholar]
- Scheuring U., Kollewe K., Haase W., Schubert D. A new method for the reconstitution of the anion transport system of the human erythrocyte membrane. J Membr Biol. 1986;90(2):123–135. doi: 10.1007/BF01869930. [DOI] [PubMed] [Google Scholar]
- Schofield A. E., Tanner M. J., Pinder J. C., Clough B., Bayley P. M., Nash G. B., Dluzewski A. R., Reardon D. M., Cox T. M., Wilson R. J. Basis of unique red cell membrane properties in hereditary ovalocytosis. J Mol Biol. 1992 Feb 20;223(4):949–958. doi: 10.1016/0022-2836(92)90254-h. [DOI] [PubMed] [Google Scholar]
- Smythe J., Gardner B., Anstee D. J. Quantitation of the number of molecules of glycophorins C and D on normal red blood cells using radioiodinated Fab fragments of monoclonal antibodies. Blood. 1994 Mar 15;83(6):1668–1672. [PubMed] [Google Scholar]
- Telen M. J., Chasis J. A. Relationship of the human erythrocyte Wrb antigen to an interaction between glycophorin A and band 3. Blood. 1990 Aug 15;76(4):842–848. [PubMed] [Google Scholar]
- Tilley L., Foley M., Anders R. F., Dluzewski A. R., Gratzer W. B., Jones G. L., Sawyer W. H. Rotational dynamics of the integral membrane protein, band 3, as a probe of the membrane events associated with Plasmodium falciparum infections of human erythrocytes. Biochim Biophys Acta. 1990 Jun 27;1025(2):135–142. doi: 10.1016/0005-2736(90)90090-b. [DOI] [PubMed] [Google Scholar]
- Tilley L., McPherson R. A., Jones G. L., Sawyer W. H. Structural organisation of band 3 in Melanesian ovalocytes. Biochim Biophys Acta. 1993 Mar 24;1181(1):83–89. doi: 10.1016/0925-4439(93)90094-h. [DOI] [PubMed] [Google Scholar]
- Tomita M., Furthmayr H., Marchesi V. T. Primary structure of human erythrocyte glycophorin A. Isolation and characterization of peptides and complete amino acid sequence. Biochemistry. 1978 Oct 31;17(22):4756–4770. doi: 10.1021/bi00615a025. [DOI] [PubMed] [Google Scholar]
- Triche T. J., Tillack T. W., Kornfeld S. Localization of the binding sites for the Ricinus communis, Agaricus bisporus and wheat germ lectins on human erythrocyte membranes. Biochim Biophys Acta. 1975 Jul 18;394(4):540–549. doi: 10.1016/0005-2736(75)90139-x. [DOI] [PubMed] [Google Scholar]
- Welsh E. J., Thom D., Morris E. R., Rees D. A. Molecular organization of glycophorin A: implications for membrane interactions. Biopolymers. 1985 Dec;24(12):2301–2332. doi: 10.1002/bip.360241210. [DOI] [PubMed] [Google Scholar]


