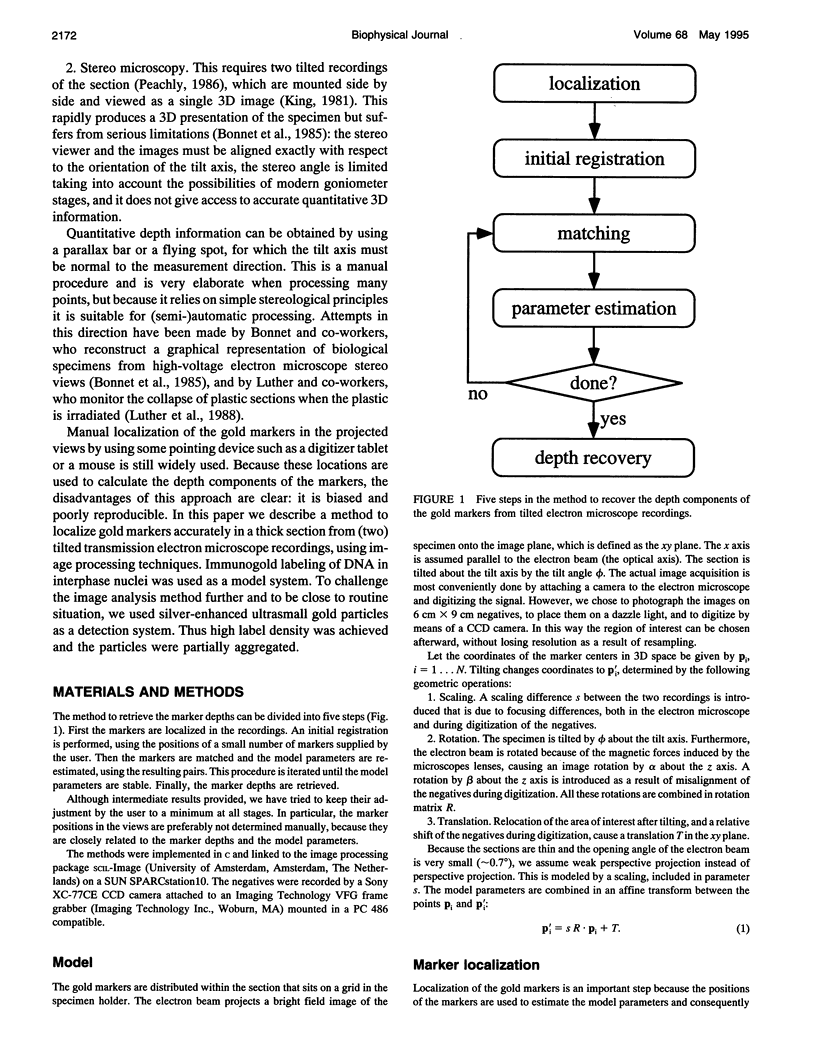
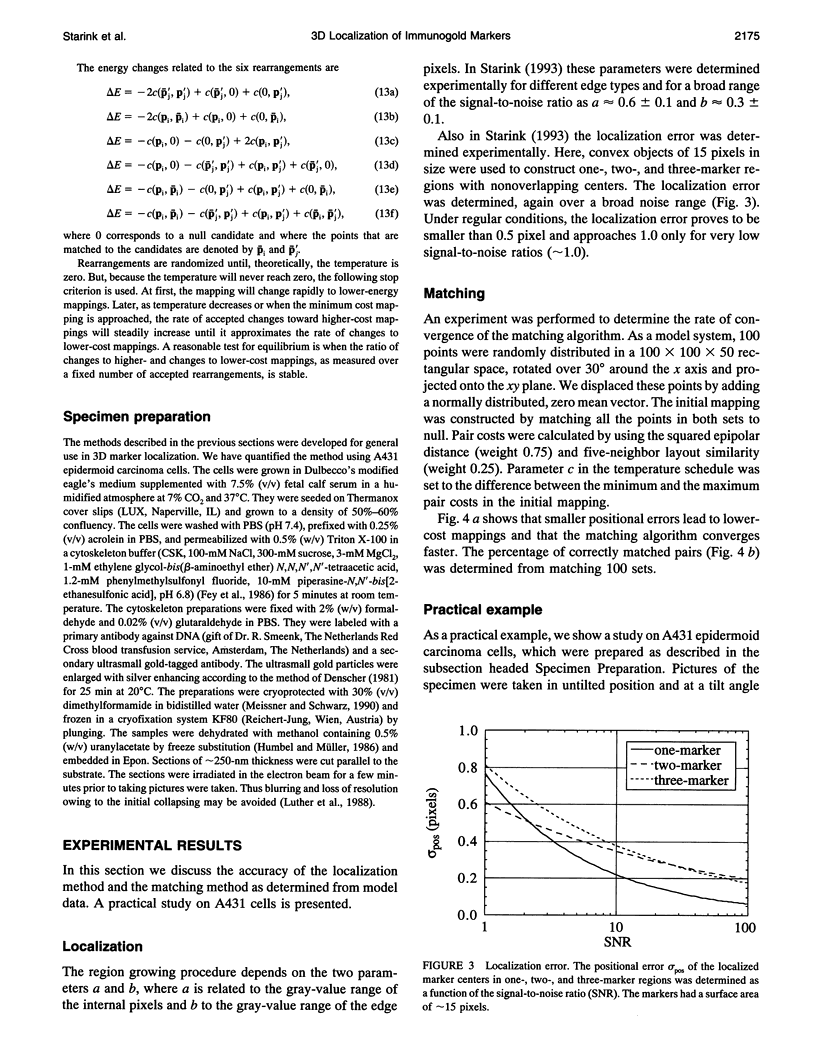
Abstract
A method is presented to determine the three-dimensional positions of immuno-labeled gold markers from tilted electron micrograph recordings by using image processing techniques. The method consists of three basic modules: localization of the markers in the recordings, estimation of the motion parameters, and matching corresponding markers between the views. Localization consists of a segmentation step based on edge detection and region growing. It also allows for the separation of (visually) aggregated markers. Initial estimates for the motion parameters are obtained from a small number of user-indicated correspondences. A matching algorithm based on simulated annealing is used to find corresponding markers. With the resulting mapping, the motion parameters are updated and used in a new matching step, etc. Once the parameters are stable, the marker depths are retrieved. The developed method has been applied to semithin resin sections of A431 cells labeled for DNA and detected by silver-enhanced ultrasmall gold particles. It represents a reliable method to analyze the three-dimensional distribution of gold markers in electron microscope samples.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baigent C. L., Müller G. Carbon-based immunocytochemistry. A new approach to the immunostaining of epoxy-resin-embedded material. J Microsc. 1990 Apr;158(Pt 1):73–80. [PubMed] [Google Scholar]
- Bonnet N., Quintana C., Favard P., Favard N. Three-dimensional graphical reconstruction from HVEM stereoviews of biological specimens by means of a microcomputer. Biol Cell. 1985;55(1-2):125–138. doi: 10.1111/j.1768-322x.1985.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Bron C., Gremillet P., Launay D., Jourlin M., Gautschi H. P., Bächi T., Schüpbach J. Three-dimensional electron microscopy of entire cells. J Microsc. 1990 Jan;157(Pt 1):115–126. doi: 10.1111/j.1365-2818.1990.tb02952.x. [DOI] [PubMed] [Google Scholar]
- Burry R. W., Lah J. J., Hayes D. M. GAP-43 distribution is correlated with development of growth cones and presynaptic terminals. J Neurocytol. 1992 Jun;21(6):413–425. doi: 10.1007/BF01191506. [DOI] [PubMed] [Google Scholar]
- Danscher G. Localization of gold in biological tissue. A photochemical method for light and electronmicroscopy. Histochemistry. 1981;71(1):81–88. doi: 10.1007/BF00592572. [DOI] [PubMed] [Google Scholar]
- Elias H. Three-dimensional structure identified from single sections. Science. 1971 Dec 3;174(4013):993–1000. doi: 10.1126/science.174.4013.993. [DOI] [PubMed] [Google Scholar]
- Fey E. G., Krochmalnic G., Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol. 1986 May;102(5):1654–1665. doi: 10.1083/jcb.102.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraud G., Soyer A., Epelboin Y. Three-dimensional computer reconstructions from serial sections of cell nuclei. Biol Cell. 1988;62(2):111–117. [PubMed] [Google Scholar]
- Horisberger M. Colloidal gold and its application in cell biology. Int Rev Cytol. 1992;136:227–287. doi: 10.1016/s0074-7696(08)62054-9. [DOI] [PubMed] [Google Scholar]
- King M. V. Theory of stereopsis. Methods Cell Biol. 1981;22:13–32. [PubMed] [Google Scholar]
- Kirkpatrick S., Gelatt C. D., Jr, Vecchi M. P. Optimization by simulated annealing. Science. 1983 May 13;220(4598):671–680. doi: 10.1126/science.220.4598.671. [DOI] [PubMed] [Google Scholar]
- Krijnse-Locker J., Ericsson M., Rottier P. J., Griffiths G. Characterization of the budding compartment of mouse hepatitis virus: evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J Cell Biol. 1994 Jan;124(1-2):55–70. doi: 10.1083/jcb.124.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leno G. H., Downes C. S., Laskey R. A. The nuclear membrane prevents replication of human G2 nuclei but not G1 nuclei in Xenopus egg extract. Cell. 1992 Apr 3;69(1):151–158. doi: 10.1016/0092-8674(92)90126-w. [DOI] [PubMed] [Google Scholar]
- Levinthal C. The formation of three-dimensional biological structures. Computer uses and future needs. Ann N Y Acad Sci. 1984;426:171–180. doi: 10.1111/j.1749-6632.1984.tb16519.x. [DOI] [PubMed] [Google Scholar]
- Luther P. K., Lawrence M. C., Crowther R. A. A method for monitoring the collapse of plastic sections as a function of electron dose. Ultramicroscopy. 1988;24(1):7–18. doi: 10.1016/0304-3991(88)90322-1. [DOI] [PubMed] [Google Scholar]
- Meissner D. H., Schwarz H. Improved cryoprotection and freeze-substitution of embryonic quail retina: a TEM study on ultrastructural preservation. J Electron Microsc Tech. 1990 Apr;14(4):348–356. doi: 10.1002/jemt.1060140410. [DOI] [PubMed] [Google Scholar]
- Moss V. A., Jenkinson D. M., Elder H. Y. Automated image segmentation and serial section reconstruction in microscopy. J Microsc. 1990 May;158(Pt 2):187–196. doi: 10.1111/j.1365-2818.1990.tb02992.x. [DOI] [PubMed] [Google Scholar]
- Nickerson J. A., Krockmalnic G., He D. C., Penman S. Immunolocalization in three dimensions: immunogold staining of cytoskeletal and nuclear matrix proteins in resinless electron microscopy sections. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2259–2263. doi: 10.1073/pnas.87.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins W. J., Green R. J. Three-dimensional reconstruction of biological sections. J Biomed Eng. 1982 Jan;4(1):37–43. doi: 10.1016/0141-5425(82)90024-3. [DOI] [PubMed] [Google Scholar]
- Provencher S. W., Vogel R. H. Three-dimensional reconstruction from electron micrographs of disordered specimens. I. Method. Ultramicroscopy. 1988;25(3):209–221. doi: 10.1016/0304-3991(88)90016-2. [DOI] [PubMed] [Google Scholar]
- Shaw P. J., Agard D. A., Hiraoka Y., Sedat J. W. Tilted view reconstruction in optical microscopy. Three-dimensional reconstruction of Drosophila melanogaster embryo nuclei. Biophys J. 1989 Jan;55(1):101–110. doi: 10.1016/S0006-3495(89)82783-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lookeren Campagne M., Dotti C. G., Jap Tjoen San E. R., Verkleij A. J., Gispen W. H., Oestreicher A. B. B-50/GAP43 localization in polarized hippocampal neurons in vitro: an ultrastructural quantitative study. Neuroscience. 1992 Sep;50(1):35–52. doi: 10.1016/0306-4522(92)90380-k. [DOI] [PubMed] [Google Scholar]
- de Graaf A., van Bergen en Henegouwen P. M., Meijne A. M., van Driel R., Verkleij A. J. Ultrastructural localization of nuclear matrix proteins in HeLa cells using silver-enhanced ultra-small gold probes. J Histochem Cytochem. 1991 Aug;39(8):1035–1045. doi: 10.1177/39.8.1856453. [DOI] [PubMed] [Google Scholar]