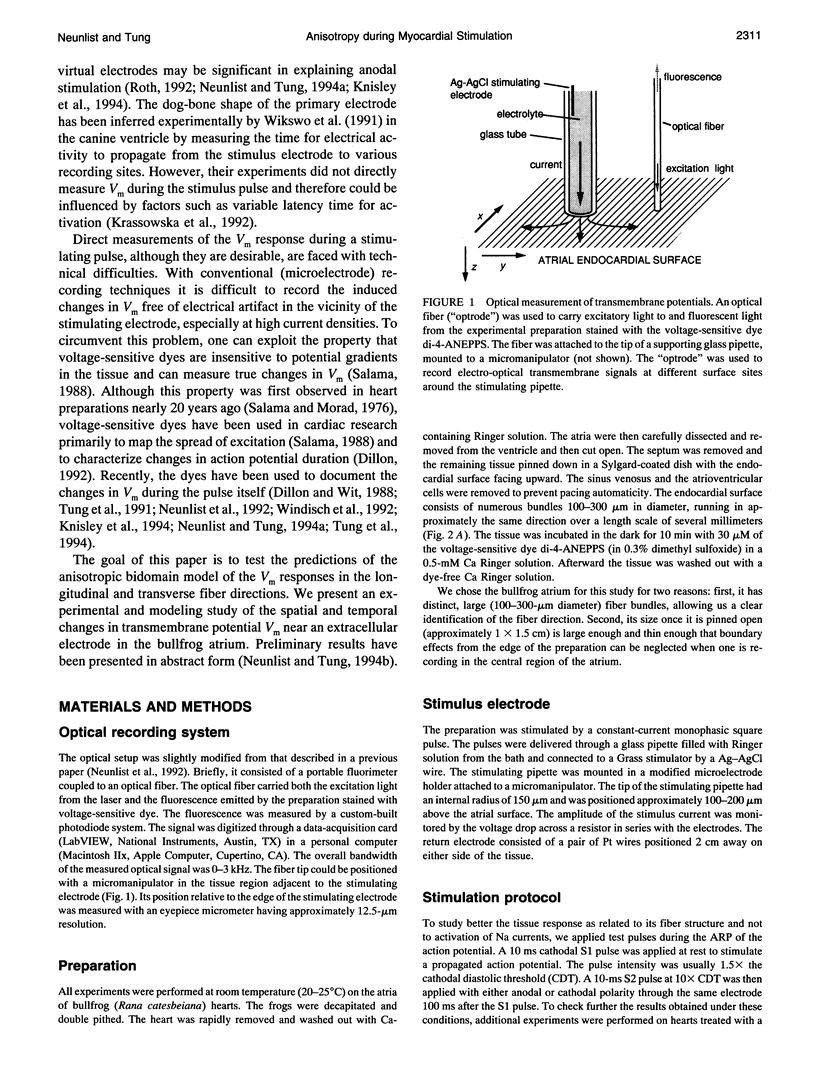
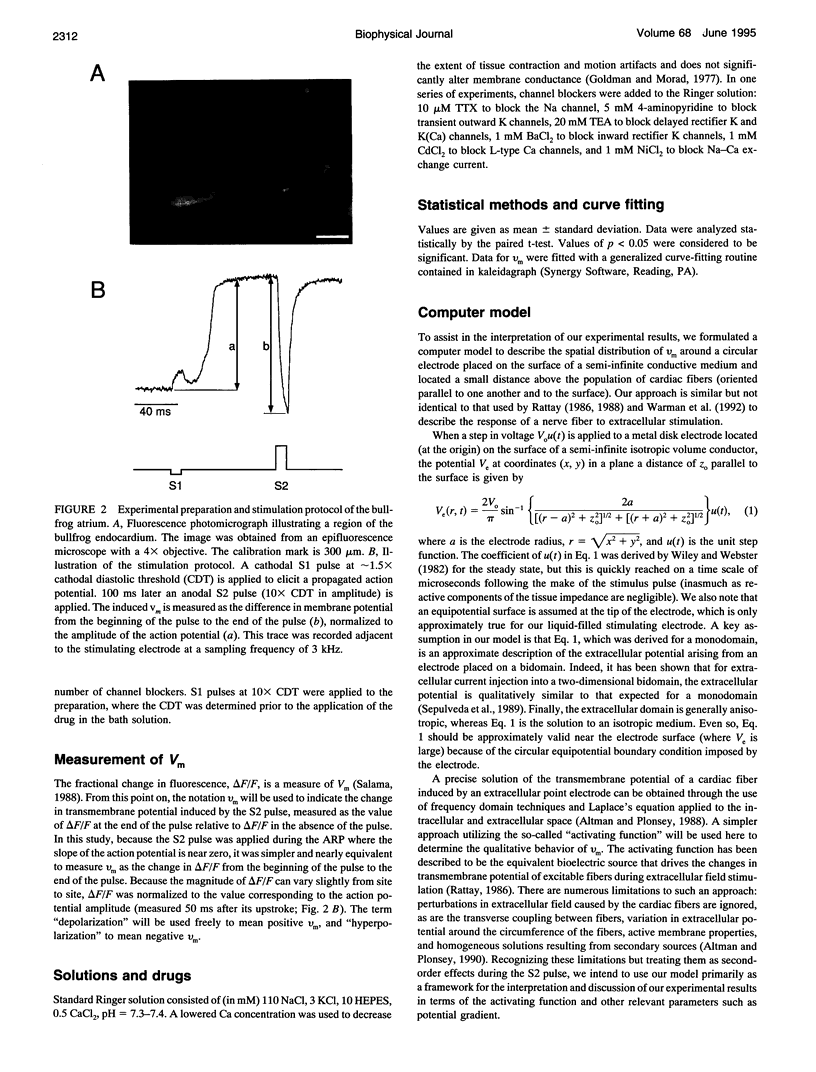
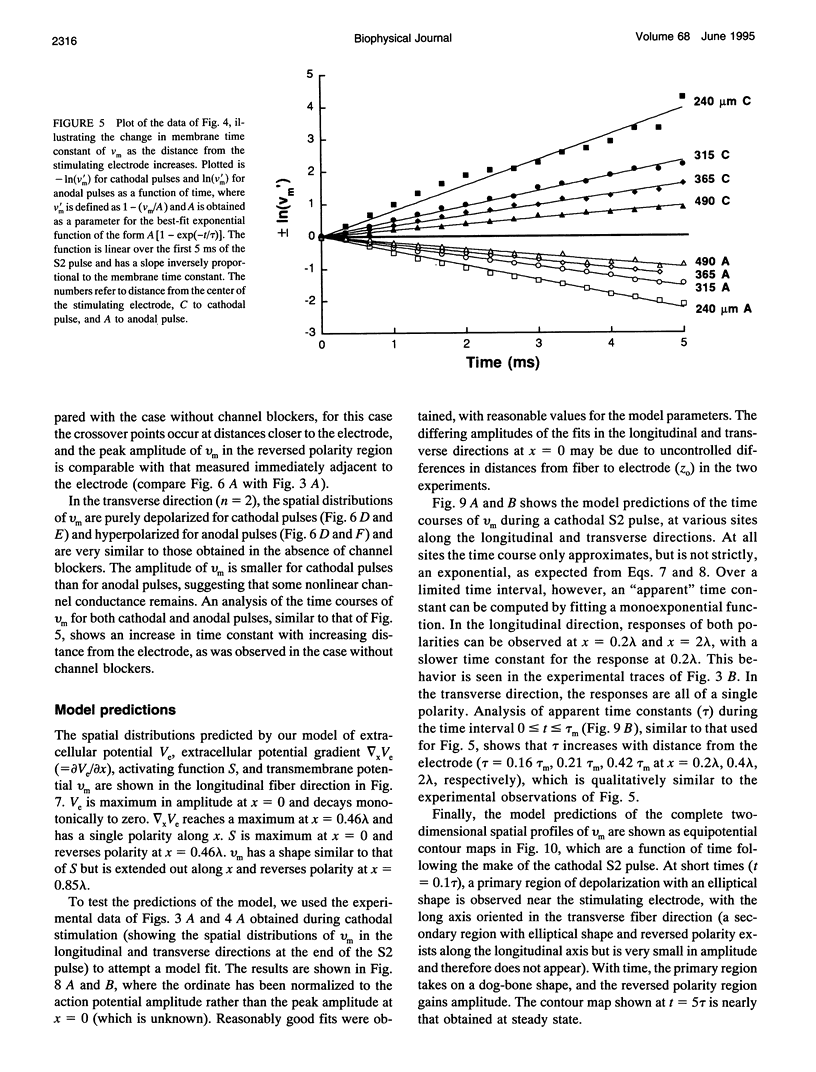
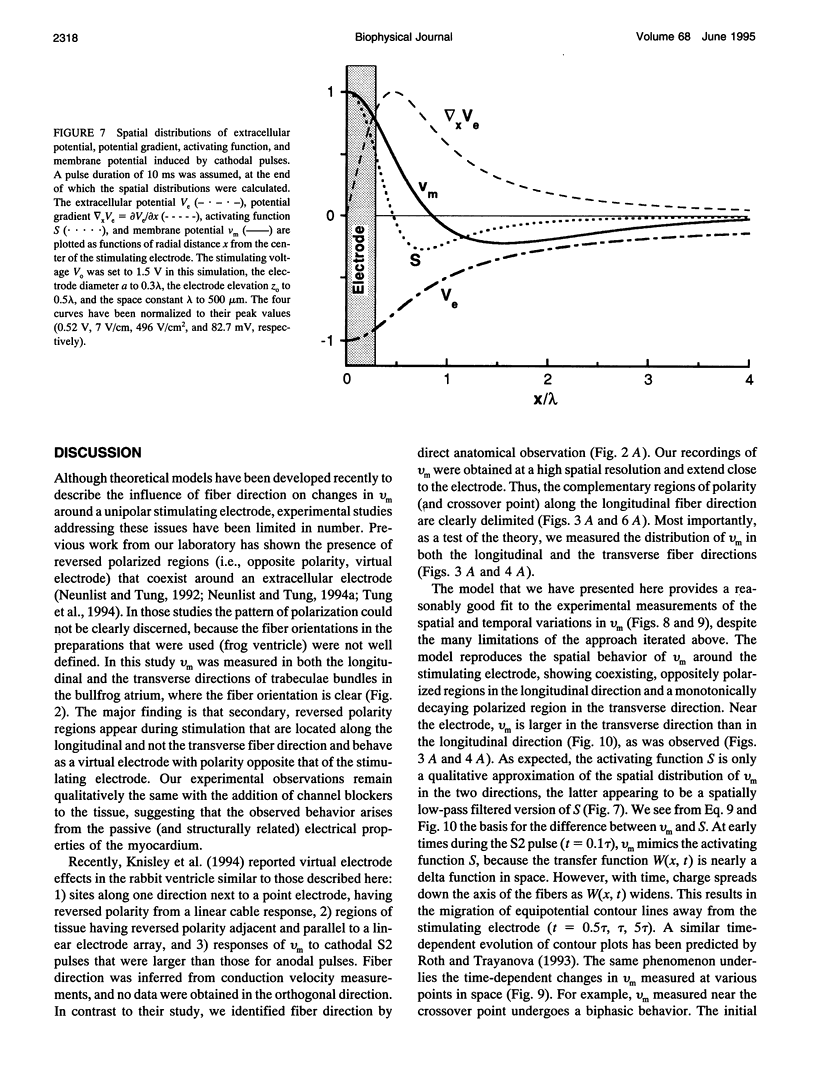
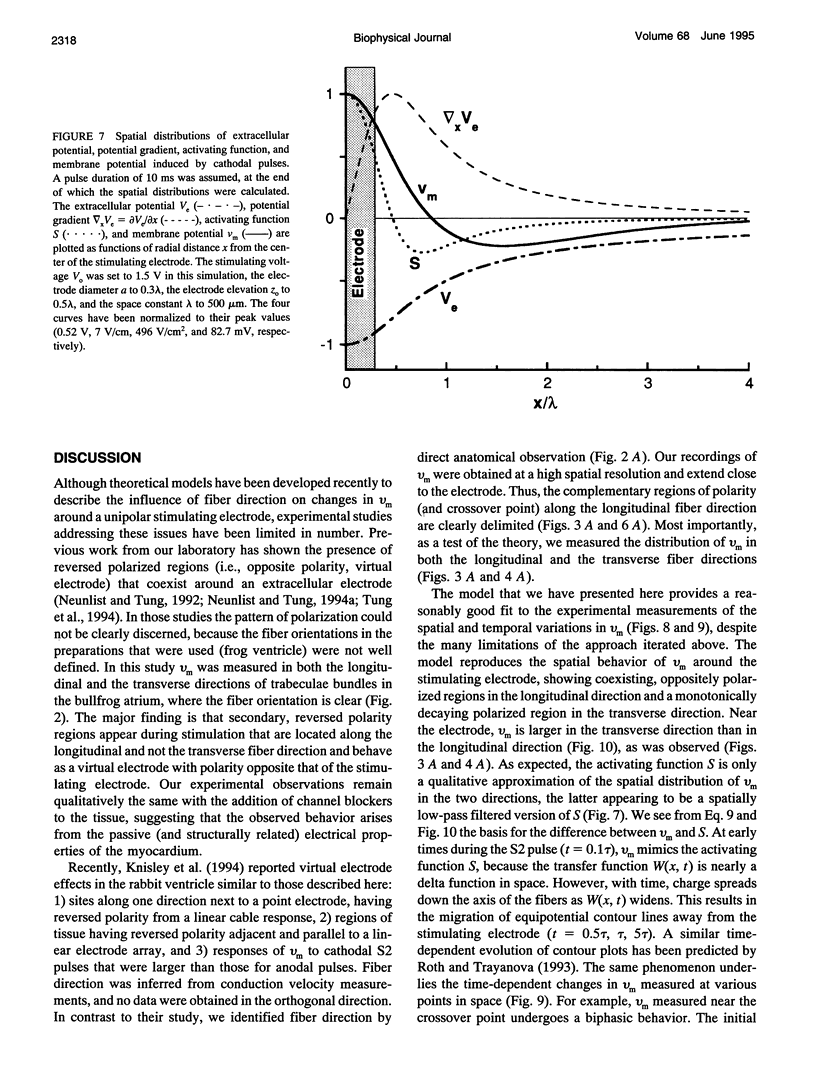
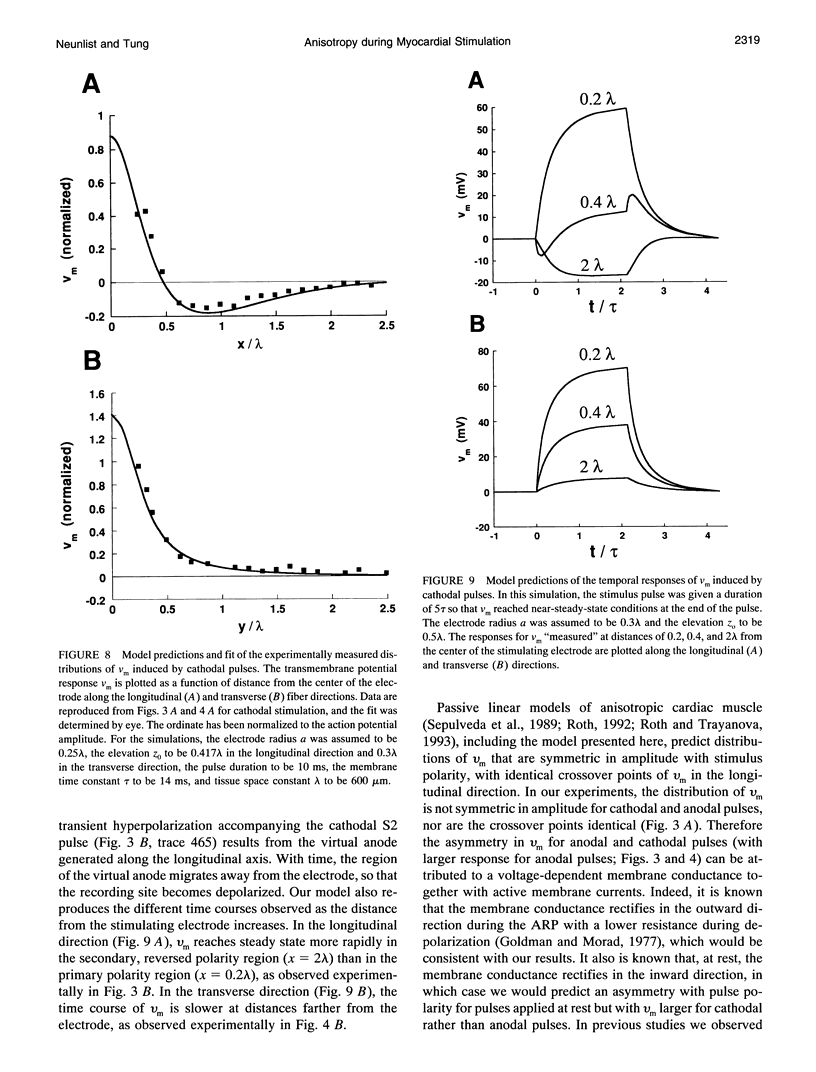
Abstract
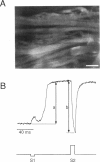
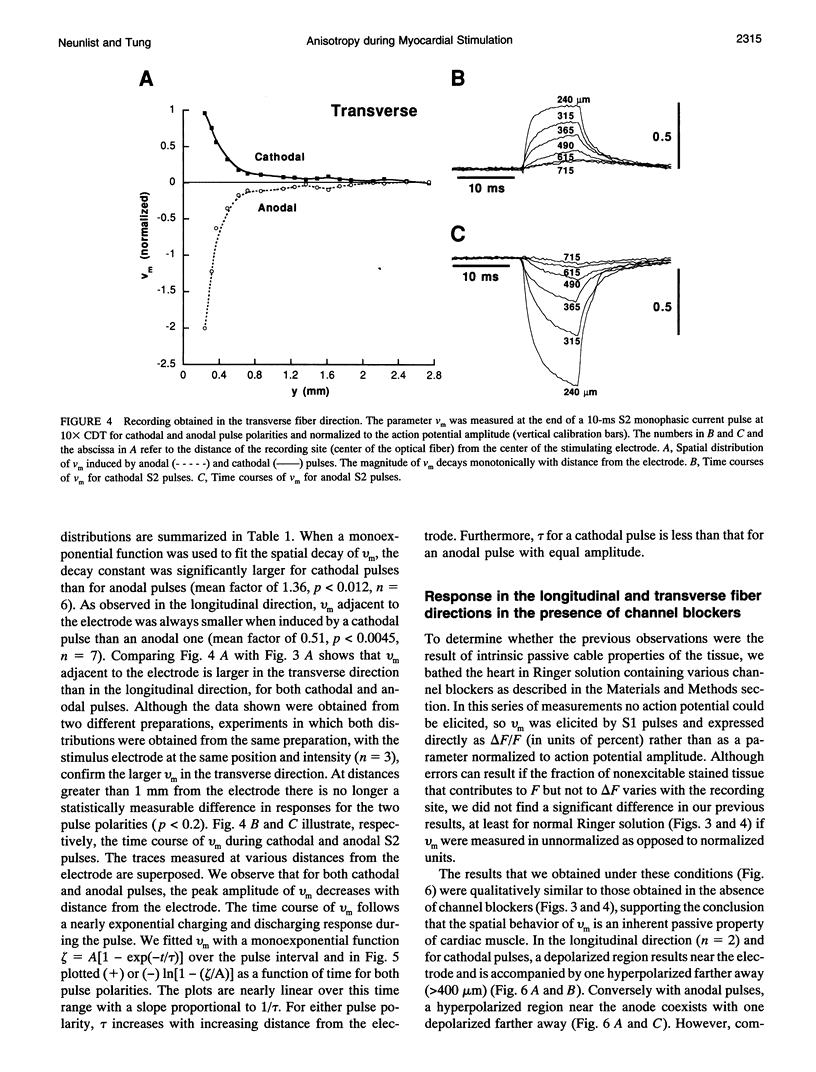
Recent theoretical models of cardiac electrical stimulation or defibrillation predict a complex spatial pattern of transmembrane potential (Vm) around a stimulating electrode, resulting from the formation of virtual electrodes of reversed polarity. The pattern of membrane polarization has been attributed to the anisotropic structure of the tissue. To verify such model predictions experimentally, an optical technique using a fluorescent voltage-sensitive dye was used to map the spatial distribution of Vm around a 150-microns-radius extracellular unipolar electrode. An S1-S2 stimulation protocol was used, and vm was measured during an S2 pulse having an intensity equal to 10x the cathodal diastolic threshold of excitation. The recordings were obtained on the endocardial surface of bullfrog atrium in directions parallel and perpendicular to the cardiac fibers. In the longitudinal fiber direction, the membrane depolarized for cathodal pulses (and hyperpolarized for anodal pulses) but only in a region within 445 +/- 112 microns (and 616 +/- 78 microns for anodal pulses) from the center of the electrode (n = 9). Outside this region, vm reversed polarity and reached a local maximum at 922 +/- 136 microns (and 988 +/- 117 microns for anodal pulses) (n = 9). Beyond this point vm decayed to zero over a distance of 1.5-2 mm. In the transverse fiber direction, the membrane depolarized for cathodal pulses (and hyperpolarized for anodal pulses) at all distances from the electrode. The amplitude of the response decreased with distance from the electrode with an exponential decay constant of 343 +/- 110 microns for cathodal pulses and 253 +/- 91 microns for anodal pulses (n = 7). The results were qualitatively similar in both fiber directions when the atrium was bathed in a solution containing ionic channel blockers. A two-dimensional computer model was formulated for the case of highly anisotropic cardiac tissue and qualitatively accounts for nearly all the observed spatial and temporal behavior of vm in the two fiber directions. The relationships between vm and both the "activating function" and extracellular potential gradient are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman K. W., Plonsey R. Analysis of excitable cell activation: relative effects of external electrical stimuli. Med Biol Eng Comput. 1990 Nov;28(6):574–580. doi: 10.1007/BF02442610. [DOI] [PubMed] [Google Scholar]
- Altman K. W., Plonsey R. Development of a model for point source electrical fibre bundle stimulation. Med Biol Eng Comput. 1988 Sep;26(5):466–475. doi: 10.1007/BF02441913. [DOI] [PubMed] [Google Scholar]
- CRANEFIELD P. F., HOFFMAN B. F., SIEBENS A. A. Anodal excitation of cardiac muscle. Am J Physiol. 1957 Aug;190(2):383–390. doi: 10.1152/ajplegacy.1957.190.2.383. [DOI] [PubMed] [Google Scholar]
- Chen P. S., Cha Y. M., Peters B. B., Chen L. S. Effects of myocardial fiber orientation on the electrical induction of ventricular fibrillation. Am J Physiol. 1993 Jun;264(6 Pt 2):H1760–H1773. doi: 10.1152/ajpheart.1993.264.6.H1760. [DOI] [PubMed] [Google Scholar]
- Dillon S. M., Allessie M. A., Ursell P. C., Wit A. L. Influences of anisotropic tissue structure on reentrant circuits in the epicardial border zone of subacute canine infarcts. Circ Res. 1988 Jul;63(1):182–206. doi: 10.1161/01.res.63.1.182. [DOI] [PubMed] [Google Scholar]
- Dillon S. M. Synchronized repolarization after defibrillation shocks. A possible component of the defibrillation process demonstrated by optical recordings in rabbit heart. Circulation. 1992 May;85(5):1865–1878. doi: 10.1161/01.cir.85.5.1865. [DOI] [PubMed] [Google Scholar]
- Frazier D. W., Krassowska W., Chen P. S., Wolf P. D., Dixon E. G., Smith W. M., Ideker R. E. Extracellular field required for excitation in three-dimensional anisotropic canine myocardium. Circ Res. 1988 Jul;63(1):147–164. doi: 10.1161/01.res.63.1.147. [DOI] [PubMed] [Google Scholar]
- Goldman Y., Morad M. Ionic membrane conductance during the time course of the cardiac action potential. J Physiol. 1977 Jul;268(3):655–695. doi: 10.1113/jphysiol.1977.sp011876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez C. S. Simulating the electrical behavior of cardiac tissue using the bidomain model. Crit Rev Biomed Eng. 1993;21(1):1–77. [PubMed] [Google Scholar]
- Knisley S. B., Blitchington T. F., Hill B. C., Grant A. O., Smith W. M., Pilkington T. C., Ideker R. E. Optical measurements of transmembrane potential changes during electric field stimulation of ventricular cells. Circ Res. 1993 Feb;72(2):255–270. doi: 10.1161/01.res.72.2.255. [DOI] [PubMed] [Google Scholar]
- Knisley S. B., Hill B. C., Ideker R. E. Virtual electrode effects in myocardial fibers. Biophys J. 1994 Mar;66(3 Pt 1):719–728. doi: 10.1016/s0006-3495(94)80846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisley S. B., Smith W. M., Ideker R. E. Effect of field stimulation on cellular repolarization in rabbit myocardium. Implications for reentry induction. Circ Res. 1992 Apr;70(4):707–715. doi: 10.1161/01.res.70.4.707. [DOI] [PubMed] [Google Scholar]
- Krassowska W., Cabo C., Knisley S. B., Ideker R. E. Propagation versus delayed activation during field stimulation of cardiac muscle. Pacing Clin Electrophysiol. 1992 Feb;15(2):197–210. doi: 10.1111/j.1540-8159.1992.tb03064.x. [DOI] [PubMed] [Google Scholar]
- Krassowska W., Frazier D. W., Pilkington T. C., Ideker R. E. Potential distribution in three-dimensional periodic myocardium--Part II: Application to extracellular stimulation. IEEE Trans Biomed Eng. 1990 Mar;37(3):267–284. doi: 10.1109/10.52328. [DOI] [PubMed] [Google Scholar]
- Lepeschkin E., Jones J. L., Rush S., Jones R. E. Local potential gradients as a unifying measure for thresholds of stimulation, standstill, tachyarrhythmia and fibrillation appearing after strong capacitor discharges. Adv Cardiol. 1978;21:268–278. doi: 10.1159/000400463. [DOI] [PubMed] [Google Scholar]
- Neunlist M., Tung L. Optical recordings of ventricular excitability of frog heart by an extracellular stimulating point electrode. Pacing Clin Electrophysiol. 1994 Oct;17(10):1641–1654. doi: 10.1111/j.1540-8159.1994.tb02359.x. [DOI] [PubMed] [Google Scholar]
- Neunlist M., Zou S. Z., Tung L. Design and use of an "optrode" for optical recordings of cardiac action potentials. Pflugers Arch. 1992 Apr;420(5-6):611–617. doi: 10.1007/BF00374641. [DOI] [PubMed] [Google Scholar]
- Osaka T., Kodama I., Tsuboi N., Toyama J., Yamada K. Effects of activation sequence and anisotropic cellular geometry on the repolarization phase of action potential of dog ventricular muscles. Circulation. 1987 Jul;76(1):226–236. doi: 10.1161/01.cir.76.1.226. [DOI] [PubMed] [Google Scholar]
- Plonsey R., Barr R. C. Electric field stimulation of excitable tissue. IEEE Trans Biomed Eng. 1995 Apr;42(4):329–336. doi: 10.1109/10.376126. [DOI] [PubMed] [Google Scholar]
- Rattay F. Analysis of models for external stimulation of axons. IEEE Trans Biomed Eng. 1986 Oct;33(10):974–977. doi: 10.1109/TBME.1986.325670. [DOI] [PubMed] [Google Scholar]
- Rattay F. Modeling the excitation of fibers under surface electrodes. IEEE Trans Biomed Eng. 1988 Mar;35(3):199–202. doi: 10.1109/10.1362. [DOI] [PubMed] [Google Scholar]
- Roberts D. E., Hersh L. T., Scher A. M. Influence of cardiac fiber orientation on wavefront voltage, conduction velocity, and tissue resistivity in the dog. Circ Res. 1979 May;44(5):701–712. doi: 10.1161/01.res.44.5.701. [DOI] [PubMed] [Google Scholar]
- Roth B. J. How the anisotropy of the intracellular and extracellular conductivities influences stimulation of cardiac muscle. J Math Biol. 1992;30(6):633–646. [PubMed] [Google Scholar]
- Roth B. J., Wikswo J. P., Jr Electrical stimulation of cardiac tissue: a bidomain model with active membrane properties. IEEE Trans Biomed Eng. 1994 Mar;41(3):232–240. doi: 10.1109/10.284941. [DOI] [PubMed] [Google Scholar]
- Salama G., Morad M. Merocyanine 540 as an optical probe of transmembrane electrical activity in the heart. Science. 1976 Feb 6;191(4226):485–487. doi: 10.1126/science.191.4226.485. [DOI] [PubMed] [Google Scholar]
- Sepulveda N. G., Roth B. J., Wikswo J. P., Jr Current injection into a two-dimensional anisotropic bidomain. Biophys J. 1989 May;55(5):987–999. doi: 10.1016/S0006-3495(89)82897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka I., Sasaki Y. On the electrotonic spread in cardiac muscle of the mouse. J Gen Physiol. 1966 Jul;49(6):1089–1110. doi: 10.1085/jgp.0491089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott G. P., Walcott K. T., Knisley S. B., Zhou X., Ideker R. E. Mechanisms of defibrillation for monophasic and biphasic waveforms. Pacing Clin Electrophysiol. 1994 Mar;17(3 Pt 2):478–498. doi: 10.1111/j.1540-8159.1994.tb01416.x. [DOI] [PubMed] [Google Scholar]
- Warman E. N., Grill W. M., Durand D. Modeling the effects of electric fields on nerve fibers: determination of excitation thresholds. IEEE Trans Biomed Eng. 1992 Dec;39(12):1244–1254. doi: 10.1109/10.184700. [DOI] [PubMed] [Google Scholar]
- Wikswo J. P., Jr, Wisialowski T. A., Altemeier W. A., Balser J. R., Kopelman H. A., Roden D. M. Virtual cathode effects during stimulation of cardiac muscle. Two-dimensional in vivo experiments. Circ Res. 1991 Feb;68(2):513–530. doi: 10.1161/01.res.68.2.513. [DOI] [PubMed] [Google Scholar]
- Wiley J. D., Webster J. G. Analysis and control of the current distribution under circular dispersive electrodes. IEEE Trans Biomed Eng. 1982 May;29(5):381–385. doi: 10.1109/TBME.1982.324910. [DOI] [PubMed] [Google Scholar]