Abstract
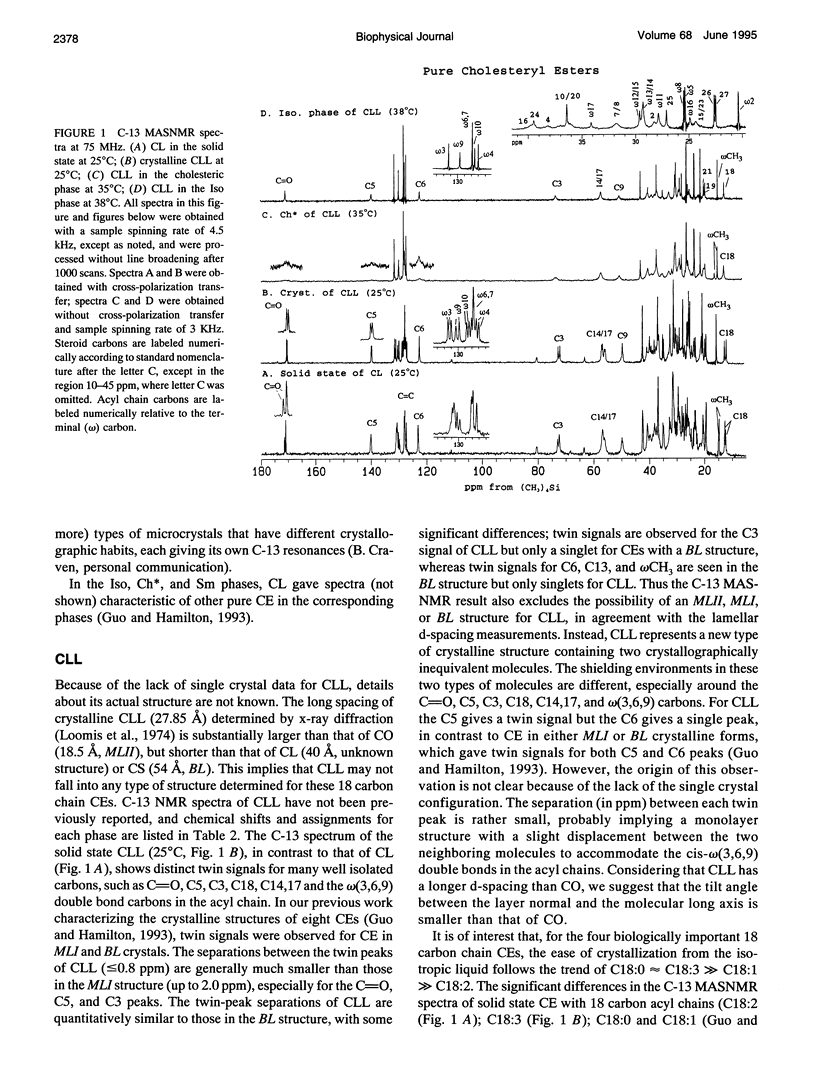
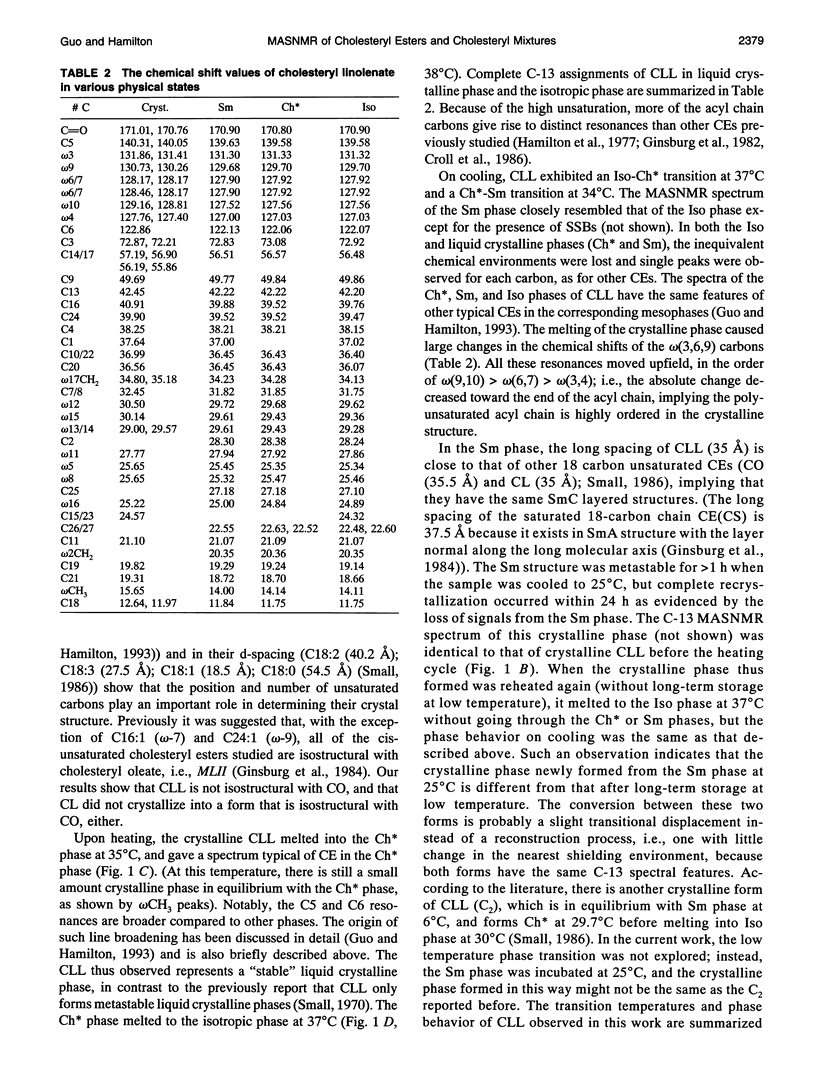
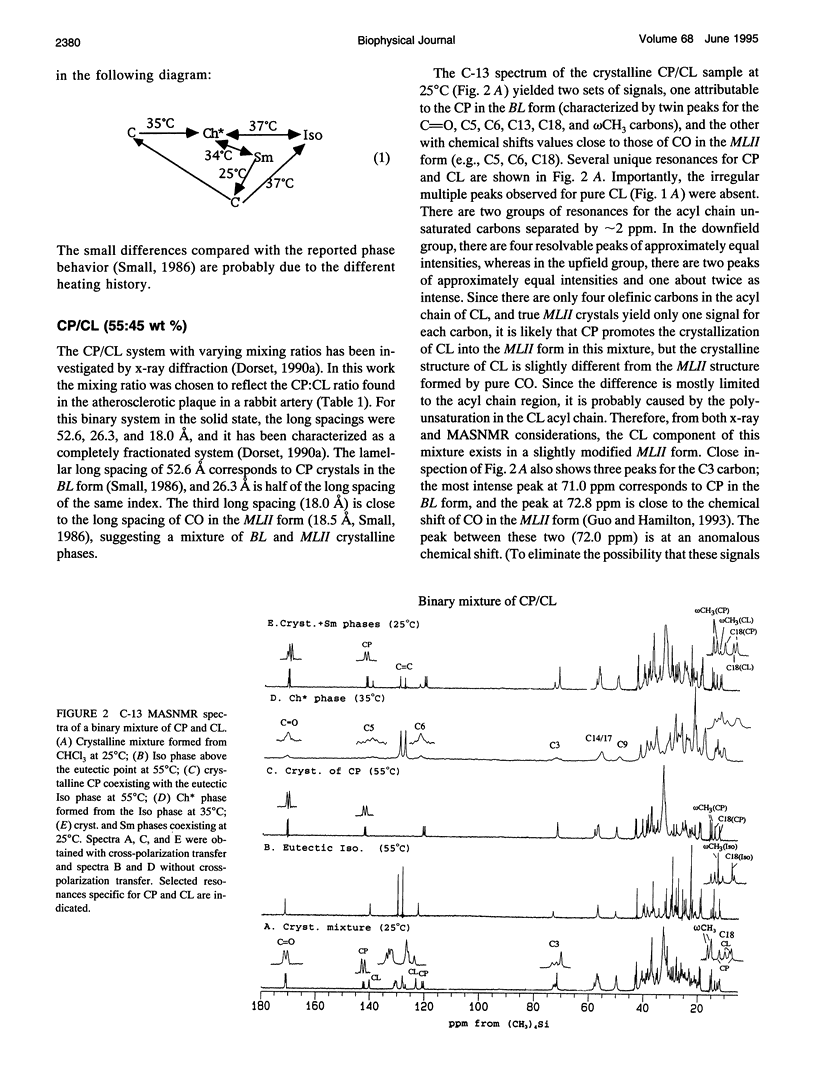
Cholesteryl esters are a transport and storage form of cholesterol in normal physiology but also a significant lipid in atherosclerotic plaques. To understand better the molecular properties of cholesteryl esters in tissues and plaques, we have studied the polymorphic and mesomorphic features of pure and mixed cholesteryl esters by solid state C-13 NMR with magic angle sample spinning (MASNMR). The temperature-dependent properties of two single components (cholesteryl linoleate (CL, C18:2) and cholesteryl linolenate (CLL, C18:3)), four binary systems (cholesteryl palmitate (CP, C16:0) with CL, CLL or cholesteryl oleate (CO, C18:1), and CO/CL), one ternary system (CO/CP/CL), and one quaternary system (CO/CP/CL/CLL) were studied. The mixing ratios were based on the composition of an atherosclerosis plaque dissected from a cholesterol-fed New Zealand white rabbit. C-13 MASNMR determined the phase transition temperatures, identified the phases present in all systems, and provided novel information about molecular structures. For example, solid CL exhibited a disordered structure with multiple molecular conformations, whereas pure CLL had a crystalline structure different from the three most commonly characterized forms (MLII, MLI, BL). In binary mixtures, the crystalline structure of each cholesteryl ester species was identified by its own characteristic resonances. It was found that CP always existed in its native BL form, but CL and CO were influenced by the composition of the mixture. CL was induced to form MLII crystals by the coexisting CP (55 wt%). When CO was cooled from the isotropic phase, it existed as a mixture of MLII and an amorphous form. The presence of CP significantly accelerated the conversion of the amorphous form to the MLII form. For the ternary mixture co-dried from chloroform, CL cocrystallized with CO in the MLII form and CP existed in BL form. Addition of a small amount of CLL slightly increased the heterogeneity of the solid mixture, but had little effect on the crystal structures or the phase transitions. C-13 MASNMR represents a powerful method for physical characterization of cholesteryl ester mixtures reflecting the composition of biological samples.
Full text
PDF

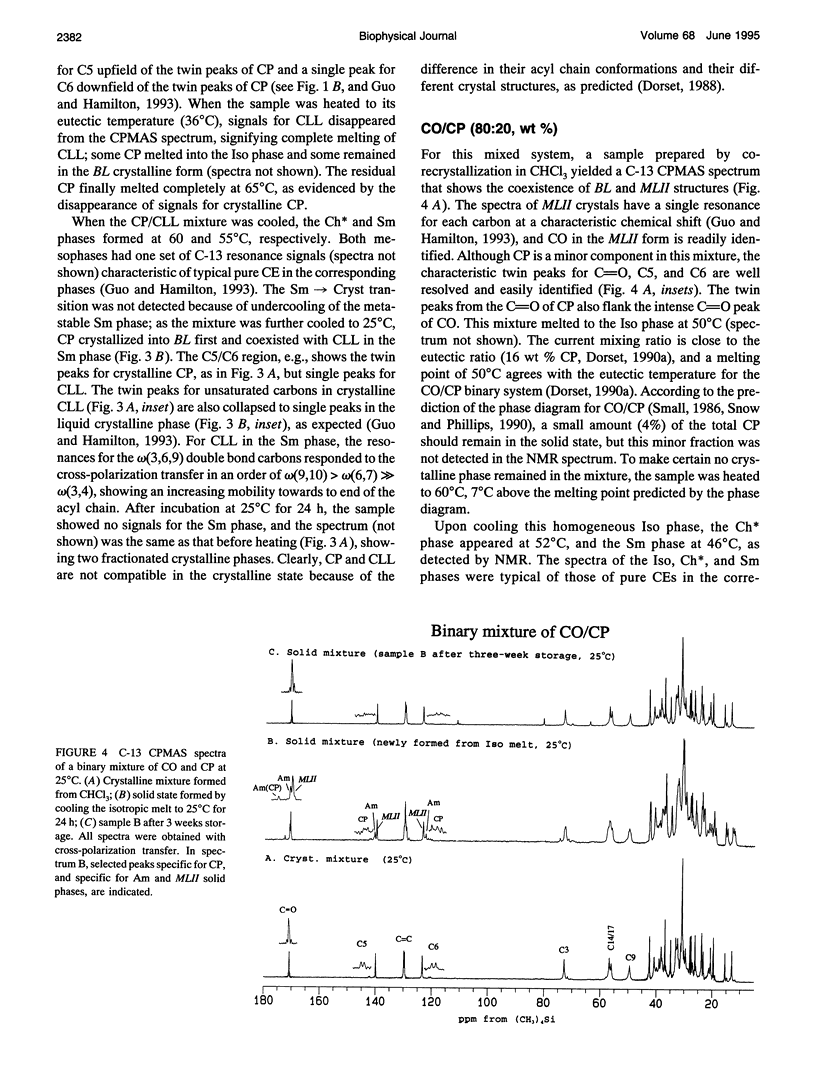
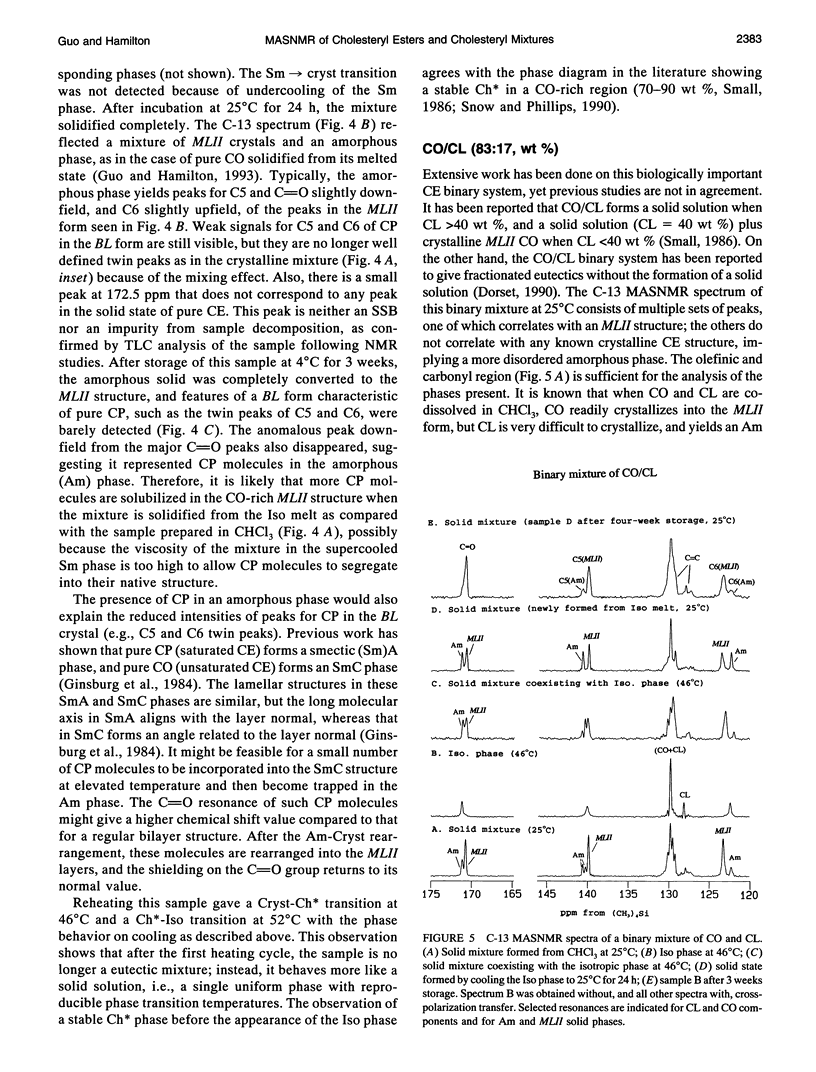
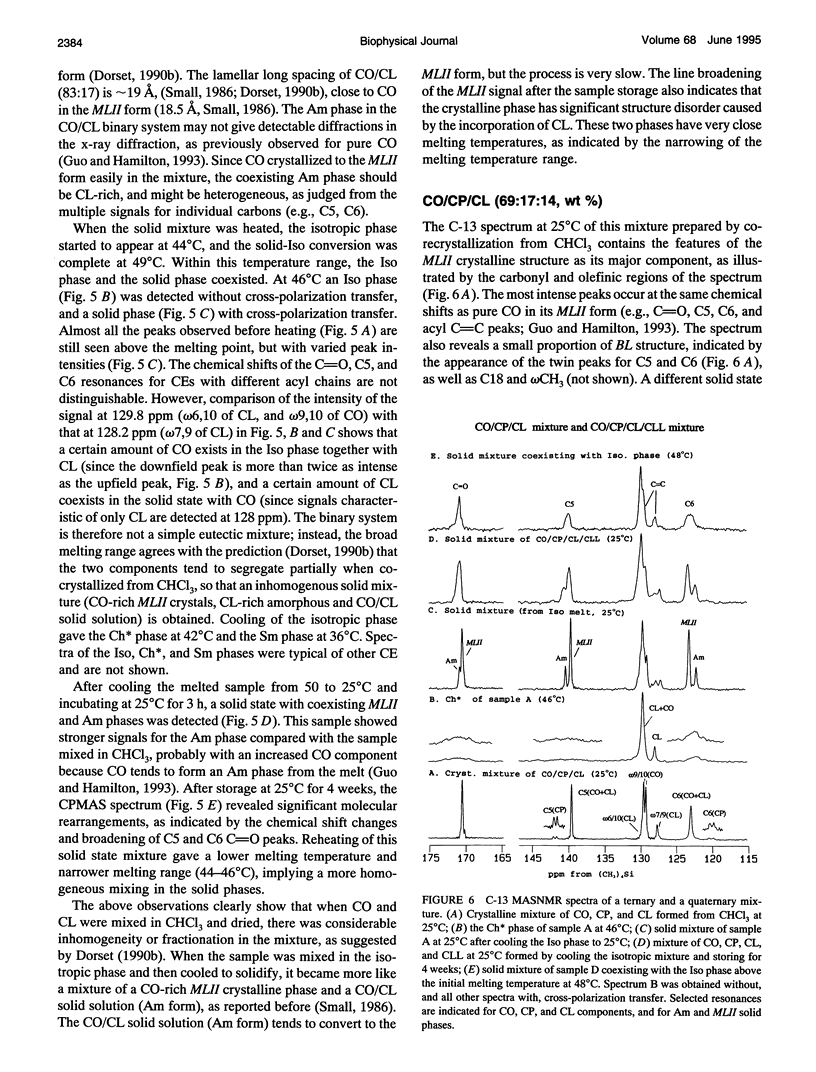


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman S. J., Glick J. M., Phillips M. C., Rothblat G. H. Lipid composition and physical state effects on cellular cholesteryl ester clearance. J Biol Chem. 1984 Nov 25;259(22):13844–13850. [PubMed] [Google Scholar]
- Burks C., Hong S., Ho M., Engelman D. M. Limitations of the lipid state hypothesis for atherosclerosis are revealed by X-ray diffraction measurements. Atherosclerosis. 1989 May;77(1):43–51. doi: 10.1016/0021-9150(89)90008-7. [DOI] [PubMed] [Google Scholar]
- Croll D. H., Small D. M., Hamilton J. A. Molecular motions and thermotropic phase behavior of cholesteryl esters with triolein. Biochemistry. 1985 Dec 31;24(27):7971–7980. doi: 10.1021/bi00348a020. [DOI] [PubMed] [Google Scholar]
- Dorset D. L. Binary phase behavior of cholesteryl oleate with cholesteryl linoleate. Biochim Biophys Acta. 1990 Aug 28;1046(1):57–63. doi: 10.1016/0005-2760(90)90094-e. [DOI] [PubMed] [Google Scholar]
- Dorset D. L. Cholesteryl esters of saturated fatty acids: cosolubility and fractionation of binary mixtures. J Lipid Res. 1987 Aug;28(8):993–1005. [PubMed] [Google Scholar]
- Dorset D. L. Co-solubility of saturated cholesteryl esters: a comparison of calculated and experimental binary phase diagrams. Biochim Biophys Acta. 1988 Nov 4;963(1):88–97. doi: 10.1016/0005-2760(88)90341-4. [DOI] [PubMed] [Google Scholar]
- Dorset D. L. Eutectic interactions between saturated and unsaturated chain cholesteryl esters: comparison of calculated and observed phase diagrams. Biochim Biophys Acta. 1990 Sep 18;1046(2):195–201. doi: 10.1016/0005-2760(90)90189-5. [DOI] [PubMed] [Google Scholar]
- Dorset D. L., Pangborn W. A. Molecular interactions in binary solids: crystal structure of a cholesteryl ester solid solution. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1822–1826. doi: 10.1073/pnas.89.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg G. S., Atkinson D., Small D. M. Physical properties of cholesteryl esters. Prog Lipid Res. 1984;23(3):135–167. doi: 10.1016/0163-7827(84)90002-x. [DOI] [PubMed] [Google Scholar]
- Ginsburg G. S., Small D. M., Hamilton J. A. Temperature-dependent molecular motions of cholesterol esters: a carbon-13 nuclear magnetic resonance study. Biochemistry. 1982 Dec 21;21(26):6857–6867. doi: 10.1021/bi00269a036. [DOI] [PubMed] [Google Scholar]
- Glick J. M., Adelman S. J. Established cell lines from rat adipose tissue that secrete lipoprotein lipase. In Vitro. 1983 May;19(5):421–428. doi: 10.1007/BF02619559. [DOI] [PubMed] [Google Scholar]
- Glick J. M., Adelman S. J., Rothblat G. H. Cholesteryl ester cycle in cultured hepatoma cells. Atherosclerosis. 1987 Apr;64(2-3):223–230. doi: 10.1016/0021-9150(87)90250-4. [DOI] [PubMed] [Google Scholar]
- Guo W., Hamilton J. A. Molecular organization and motions of cholesteryl esters in crystalline and liquid crystalline phases: a 13C and 1H magic angle spinning NMR study. Biochemistry. 1993 Sep 7;32(35):9038–9052. doi: 10.1021/bi00086a009. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Cordes E. H., Glueck C. J. Lipid dynamics in human low density lipoproteins and human aortic tissue with fibrous plaques. A study by high field 13C NMR spectroscopy. J Biol Chem. 1979 Jun 25;254(12):5435–5441. [PubMed] [Google Scholar]
- Hamilton J. A., Oppenheimer N., Cordes E. H. Carbon-13 nuclear magnetic resonance studies of cholesteryl esters and cholesteryl ester/triglyceride mixtures. J Biol Chem. 1977 Nov 25;252(22):8071–8080. [PubMed] [Google Scholar]
- Loomis C. R., Janiak M. J., Small D. M., Shipley G. G. The binary phase diagram of lecithin and cholesteryl linolenate. J Mol Biol. 1974 Jun 25;86(2):309–324. doi: 10.1016/0022-2836(74)90021-7. [DOI] [PubMed] [Google Scholar]
- Lundberg B. B., Rothblat G. H., Glick J. M., Phillips M. C. Effect of substrate physical state on the activity of acid cholesteryl ester hydrolase. Biochim Biophys Acta. 1990 Feb 23;1042(3):301–309. doi: 10.1016/0005-2760(90)90157-s. [DOI] [PubMed] [Google Scholar]
- McCourt M. P., Strong P., Pangborn W., Dorset D. L. Structural determination and packing analysis of a cholesteryl caprate/cholesteryl laurate solid solution. J Lipid Res. 1994 Apr;35(4):584–591. [PubMed] [Google Scholar]
- Phillips M. C., Johnson W. J., Rothblat G. H. Mechanisms and consequences of cellular cholesterol exchange and transfer. Biochim Biophys Acta. 1987 Jun 24;906(2):223–276. doi: 10.1016/0304-4157(87)90013-x. [DOI] [PubMed] [Google Scholar]
- Small D. M., Bond M. G., Waugh D., Prack M., Sawyer J. K. Physicochemical and histological changes in the arterial wall of nonhuman primates during progression and regression of atherosclerosis. J Clin Invest. 1984 Jun;73(6):1590–1605. doi: 10.1172/JCI111366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D. M. George Lyman Duff memorial lecture. Progression and regression of atherosclerotic lesions. Insights from lipid physical biochemistry. Arteriosclerosis. 1988 Mar-Apr;8(2):103–129. doi: 10.1161/01.atv.8.2.103. [DOI] [PubMed] [Google Scholar]
- Snow J., Phillips M. C. Phase behavior of cholesteryl ester dispersions which model the inclusions of foam cells. Biochemistry. 1990 Mar 13;29(10):2464–2471. doi: 10.1021/bi00462a005. [DOI] [PubMed] [Google Scholar]


