Abstract
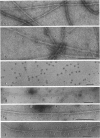
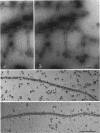
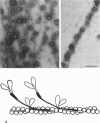
The structure of smooth muscle thin filament was examined by various electron microscopy techniques, with special attention to the mode of caldesmon binding. Chemical cross-linking was positively used to avoid the dissociation of accessory proteins upon dilution. Caldesmon in reconstituted thin filament was observed as fine filamentous projections from thin filament. Native thin filament isolated from smooth muscle showed similarly numerous fine whisker-like projections by all the techniques employed here. Antibody against the amino-terminus of caldesmon labeled the end of such projections indicating the possibility that the amino-terminal myosin binding moiety might stick out from the shaft of the thin filament. Such whiskers are often projected out as a cluster to the same side of native thin filament. Further, we could visualize the assembly of dephosphorylated heavy meromyosin (HMM) with native or reconstituted thin filament forming "nonproductive" complex in the presence of ATP. The association of HMM to the shaft of thin filament was through subfragment-2 moiety, in accordance with biochemical studies. Some HMM particles bound closer to the thin filament shaft, possibly suggesting the presence of the second myosin-binding site on caldesmon. Occasionally two kinds of HMM association as such coexisted at a single site on this filament in tandem. Thus, we constructed a structural model of thin filament. The proposed molecular arrangement is not only compatible with all the biochemical results but also provides additional support for our recent findings (E. Katayoma, G. C. Scott-Woo, and M. Ikebe (1995) J. Biol. Chem. 270, 3919-3925) regarding the capability of caldesmon to induce dephosphorylated myosin filament, which explains the existence of thick filaments in relaxed smooth muscle cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki Y., Ikebe M. Activation of smooth muscle myosin light chain kinase activity by a monoclonal antibody which recognizes the calmodulin-binding region. Biochem J. 1991 May 1;275(Pt 3):679–684. doi: 10.1042/bj2750679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J., Imai M., Lee R., Moore P., Cook R. G., Lin W. G. Cloning and expression of a smooth muscle caldesmon. J Biol Chem. 1989 Aug 15;264(23):13873–13879. [PubMed] [Google Scholar]
- Cooke P. H., Fay F. S., Craig R. Myosin filaments isolated from skinned amphibian smooth muscle cells are side-polar. J Muscle Res Cell Motil. 1989 Jun;10(3):206–220. doi: 10.1007/BF01739811. [DOI] [PubMed] [Google Scholar]
- Craig R., Megerman J. Assembly of smooth muscle myosin into side-polar filaments. J Cell Biol. 1977 Dec;75(3):990–996. doi: 10.1083/jcb.75.3.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingus J., Hwo S., Bryan J. Identification by monoclonal antibodies and characterization of human platelet caldesmon. J Cell Biol. 1986 May;102(5):1748–1757. doi: 10.1083/jcb.102.5.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Imai M., Rosenfeld G. C., Bryan J. Domain mapping of chicken gizzard caldesmon. J Biol Chem. 1987 Feb 25;262(6):2757–2763. [PubMed] [Google Scholar]
- Fürst D. O., Cross R. A., De Mey J., Small J. V. Caldesmon is an elongated, flexible molecule localized in the actomyosin domains of smooth muscle. EMBO J. 1986 Feb;5(2):251–257. doi: 10.1002/j.1460-2075.1986.tb04206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graceffa P., Adam L. P., Lehman W. Disulphide cross-linking of smooth-muscle and non-muscle caldesmon to the C-terminus of actin in reconstituted and native thin filaments. Biochem J. 1993 Aug 15;294(Pt 1):63–67. doi: 10.1042/bj2940063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graceffa P., Wang C. L., Stafford W. F. Caldesmon. Molecular weight and subunit composition by analytical ultracentrifugation. J Biol Chem. 1988 Oct 5;263(28):14196–14202. [PubMed] [Google Scholar]
- Hayashi K., Kanda K., Kimizuka F., Kato I., Sobue K. Primary structure and functional expression of h-caldesmon complementary DNA. Biochem Biophys Res Commun. 1989 Oct 16;164(1):503–511. doi: 10.1016/0006-291x(89)91748-8. [DOI] [PubMed] [Google Scholar]
- Hemric M. E., Chalovich J. M. Characterization of caldesmon binding to myosin. J Biol Chem. 1990 Nov 15;265(32):19672–19678. [PMC free article] [PubMed] [Google Scholar]
- Hemric M. E., Chalovich J. M. Effect of caldesmon on the ATPase activity and the binding of smooth and skeletal myosin subfragments to actin. J Biol Chem. 1988 Feb 5;263(4):1878–1885. [PubMed] [Google Scholar]
- Heuser J. E. Procedure for freeze-drying molecules adsorbed to mica flakes. J Mol Biol. 1983 Sep 5;169(1):155–195. doi: 10.1016/s0022-2836(83)80179-x. [DOI] [PubMed] [Google Scholar]
- Highashihara M., Frado L. L., Craig R., Ikebe M. Inhibition of conformational change in smooth muscle myosin by a monoclonal antibody against the 17-kDa light chain. J Biol Chem. 1989 Mar 25;264(9):5218–5225. [PubMed] [Google Scholar]
- Horiuchi K. Y., Chacko S. Interaction between caldesmon and tropomyosin in the presence and absence of smooth muscle actin. Biochemistry. 1988 Nov 1;27(22):8388–8393. doi: 10.1021/bi00422a014. [DOI] [PubMed] [Google Scholar]
- Huber P. A., Redwood C. S., Avent N. D., Tanner M. J., Marston S. B. Identification of functioning regulatory sites and a new myosin binding site in the C-terminal 288 amino acids of caldesmon expressed from a human clone. J Muscle Res Cell Motil. 1993 Aug;14(4):385–391. doi: 10.1007/BF00121289. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Reardon S. Binding of caldesmon to smooth muscle myosin. J Biol Chem. 1988 Mar 5;263(7):3055–3058. [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. Regulation of smooth muscle contractile elements by second messengers. Annu Rev Physiol. 1989;51:299–313. doi: 10.1146/annurev.ph.51.030189.001503. [DOI] [PubMed] [Google Scholar]
- Katayama E. Assignment of the positions of chymotryptic fragments and cysteinyl groups in the primary structure of caldesmon in relation to a conformational change. J Biochem. 1989 Dec;106(6):988–993. doi: 10.1093/oxfordjournals.jbchem.a122987. [DOI] [PubMed] [Google Scholar]
- Katayama E., Horiuchi K. Y., Chacko S. Characteristics of the myosin and tropomyosin binding regions of the smooth muscle caldesmon. Biochem Biophys Res Commun. 1989 May 15;160(3):1316–1322. doi: 10.1016/s0006-291x(89)80147-0. [DOI] [PubMed] [Google Scholar]
- Katayama E., Scott-Woo G., Ikebe M. Effect of caldesmon on the assembly of smooth muscle myosin. J Biol Chem. 1995 Feb 24;270(8):3919–3925. doi: 10.1074/jbc.270.8.3919. [DOI] [PubMed] [Google Scholar]
- Katayama E. The effects of various nucleotides on the structure of actin-attached myosin subfragment-1 studied by quick-freeze deep-etch electron microscopy. J Biochem. 1989 Nov;106(5):751–770. doi: 10.1093/oxfordjournals.jbchem.a122928. [DOI] [PubMed] [Google Scholar]
- Lash J. A., Sellers J. R., Hathaway D. R. The effects of caldesmon on smooth muscle heavy actomeromyosin ATPase activity and binding of heavy meromyosin to actin. J Biol Chem. 1986 Dec 5;261(34):16155–16160. [PubMed] [Google Scholar]
- Lehman W., Craig R., Lui J., Moody C. Caldesmon and the structure of smooth muscle thin filaments: immunolocalization of caldesmon on thin filaments. J Muscle Res Cell Motil. 1989 Apr;10(2):101–112. doi: 10.1007/BF01739966. [DOI] [PubMed] [Google Scholar]
- Lehman W., Moody C., Craig R. Caldesmon and the structure of vertebrate smooth muscle thin filaments. A minireview. Ann N Y Acad Sci. 1990;599:75–84. doi: 10.1111/j.1749-6632.1990.tb42366.x. [DOI] [PubMed] [Google Scholar]
- Lynch W. P., Riseman V. M., Bretscher A. Smooth muscle caldesmon is an extended flexible monomeric protein in solution that can readily undergo reversible intra- and intermolecular sulfhydryl cross-linking. A mechanism for caldesmon's F-actin bundling activity. J Biol Chem. 1987 May 25;262(15):7429–7437. [PubMed] [Google Scholar]
- Mabuchi K. Heavy-meromyosin-decorated actin filaments: a simple method to preserve actin filaments for rotary shadowing. J Struct Biol. 1991 Aug;107(1):22–28. doi: 10.1016/1047-8477(91)90027-t. [DOI] [PubMed] [Google Scholar]
- Mabuchi K., Lin J. J., Wang C. L. Electron microscopic images suggest both ends of caldesmon interact with actin filaments. J Muscle Res Cell Motil. 1993 Feb;14(1):54–64. doi: 10.1007/BF00132180. [DOI] [PubMed] [Google Scholar]
- Mabuchi K., Wang C. L. Electron microscopic studies of chicken gizzard caldesmon and its complex with calmodulin. J Muscle Res Cell Motil. 1991 Apr;12(2):145–151. doi: 10.1007/BF01774033. [DOI] [PubMed] [Google Scholar]
- Marston S. B. A tight-binding interaction between smooth-muscle native thin filaments and heavy meromyosin in the presence of MgATP. Biochem J. 1989 Apr 1;259(1):303–306. doi: 10.1042/bj2590303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B., Lehman W. Caldesmon is a Ca2+-regulatory component of native smooth-muscle thin filaments. Biochem J. 1985 Nov 1;231(3):517–522. doi: 10.1042/bj2310517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B., Redwood C. S. The molecular anatomy of caldesmon. Biochem J. 1991 Oct 1;279(Pt 1):1–16. doi: 10.1042/bj2790001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S., Pinter K., Bennett P. Caldesmon binds to smooth muscle myosin and myosin rod and crosslinks thick filaments to actin filaments. J Muscle Res Cell Motil. 1992 Apr;13(2):206–218. doi: 10.1007/BF01874158. [DOI] [PubMed] [Google Scholar]
- Marston S. Stoichiometry and stability of caldesmon in native thin filaments from sheep aorta smooth muscle. Biochem J. 1990 Dec 1;272(2):305–310. doi: 10.1042/bj2720305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody C., Lehman W., Craig R. Caldesmon and the structure of smooth muscle thin filaments: electron microscopy of isolated thin filaments. J Muscle Res Cell Motil. 1990 Apr;11(2):176–185. doi: 10.1007/BF01766496. [DOI] [PubMed] [Google Scholar]
- Prochniewicz E., Katayama E., Yanagida T., Thomas D. D. Cooperativity in F-actin: chemical modifications of actin monomers affect the functional interactions of myosin with unmodified monomers in the same actin filament. Biophys J. 1993 Jul;65(1):113–123. doi: 10.1016/S0006-3495(93)81057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwood C. S., Marston S. B. Binding and regulatory properties of expressed functional domains of chicken gizzard smooth muscle caldesmon. J Biol Chem. 1993 May 25;268(15):10969–10976. [PubMed] [Google Scholar]
- SZENT-GYORGYI A. G. A new method for the preparation of actin. J Biol Chem. 1951 Sep;192(1):361–369. [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5652–5655. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K., Sellers J. R. Caldesmon, a novel regulatory protein in smooth muscle and nonmuscle actomyosin systems. J Biol Chem. 1991 Jul 5;266(19):12115–12118. [PubMed] [Google Scholar]
- Somlyo A. V., Butler T. M., Bond M., Somlyo A. P. Myosin filaments have non-phosphorylated light chains in relaxed smooth muscle. Nature. 1981 Dec 10;294(5841):567–569. doi: 10.1038/294567a0. [DOI] [PubMed] [Google Scholar]
- Sutherland C., Walsh M. P. Phosphorylation of caldesmon prevents its interaction with smooth muscle myosin. J Biol Chem. 1989 Jan 5;264(1):578–583. [PubMed] [Google Scholar]
- Szpacenko A., Dabrowska R. Functional domain of caldesmon. FEBS Lett. 1986 Jul 7;202(2):182–186. doi: 10.1016/0014-5793(86)80683-4. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Tsukita S., Usukura J., Ishikawa H. Myosin filaments in smooth muscle cells of the guinea pig taenia coli: a freeze-substitution study. Eur J Cell Biol. 1982 Oct;28(2):195–201. [PubMed] [Google Scholar]
- Velaz L., Hemric M. E., Benson C. E., Chalovich J. M. The binding of caldesmon to actin and its effect on the ATPase activity of soluble myosin subfragments in the presence and absence of tropomyosin. J Biol Chem. 1989 Jun 5;264(16):9602–9610. [PubMed] [Google Scholar]
- Velaz L., Ingraham R. H., Chalovich J. M. Dissociation of the effect of caldesmon on the ATPase activity and on the binding of smooth heavy meromyosin to actin by partial digestion of caldesmon. J Biol Chem. 1990 Feb 15;265(5):2929–2934. [PubMed] [Google Scholar]
- Vibert P., Craig R., Lehman W. Three-dimensional reconstruction of caldesmon-containing smooth muscle thin filaments. J Cell Biol. 1993 Oct;123(2):313–321. doi: 10.1083/jcb.123.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. L., Chalovich J. M., Graceffa P., Lu R. C., Mabuchi K., Stafford W. F. A long helix from the central region of smooth muscle caldesmon. J Biol Chem. 1991 Jul 25;266(21):13958–13963. [PMC free article] [PubMed] [Google Scholar]
- Yazawa M., Yagi K., Sobue K. Isolation and characterization of a calmodulin binding fragment of chicken gizzard caldesmon. J Biochem. 1987 Nov;102(5):1065–1073. doi: 10.1093/oxfordjournals.jbchem.a122144. [DOI] [PubMed] [Google Scholar]