Abstract
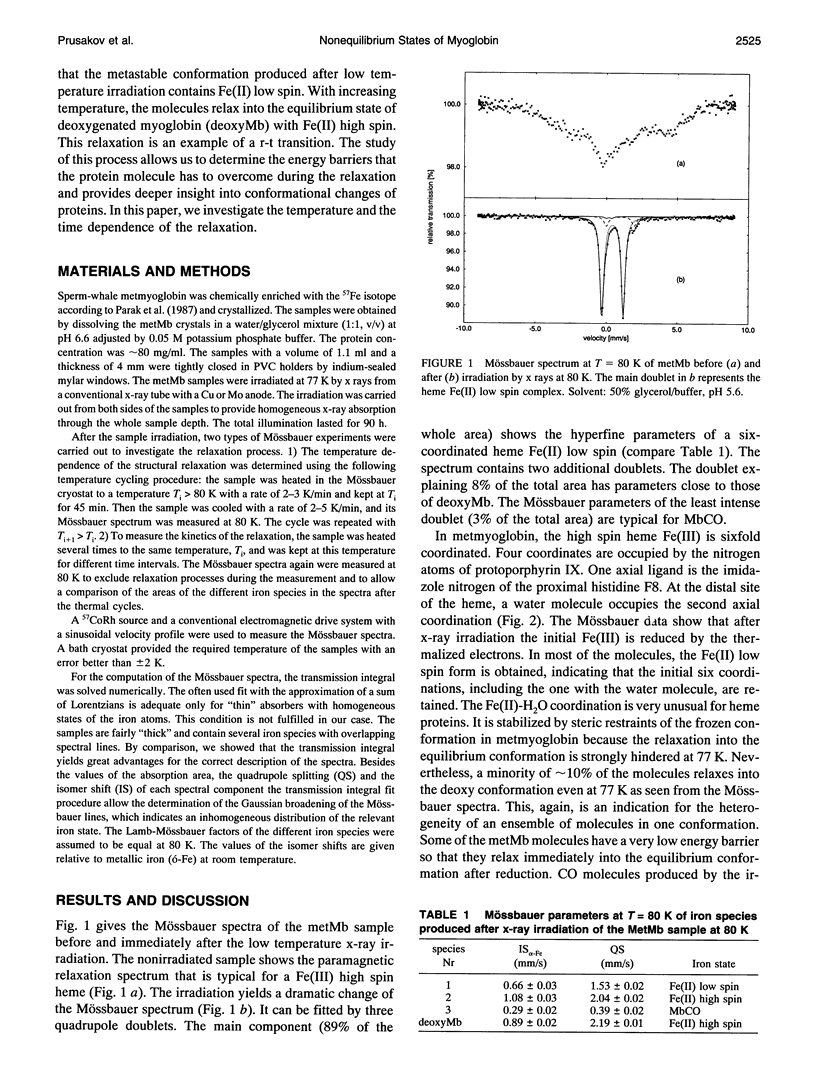
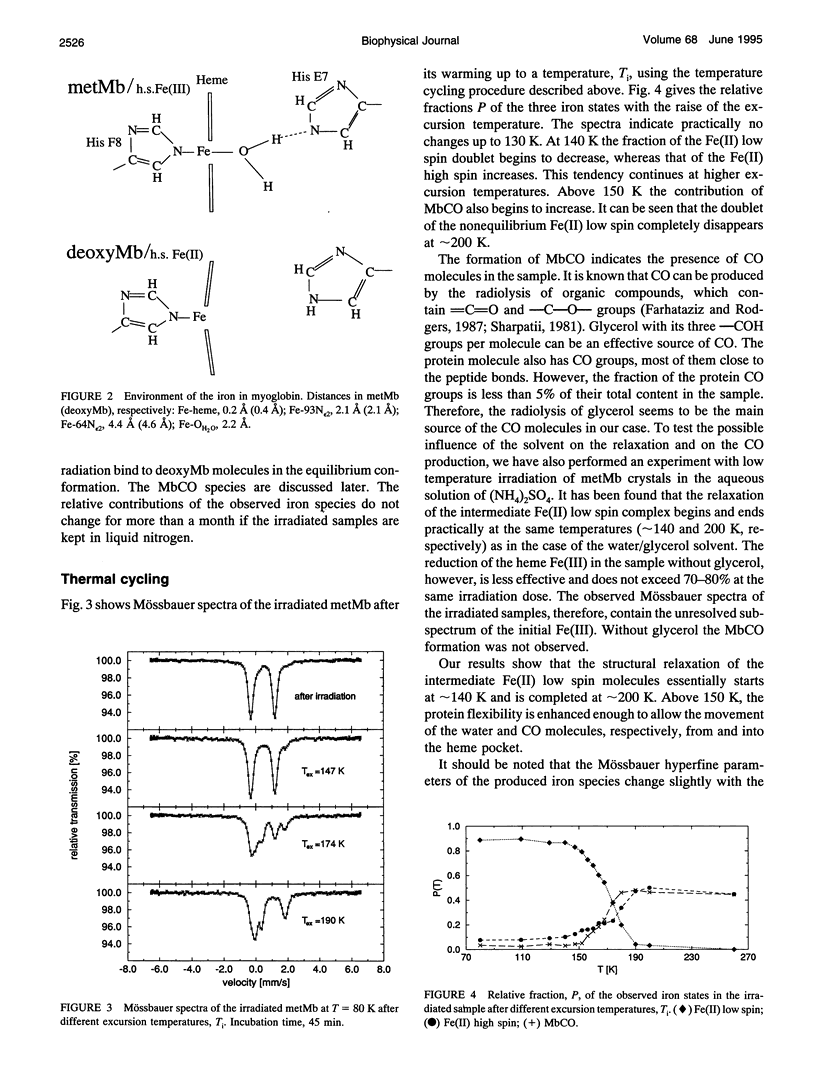
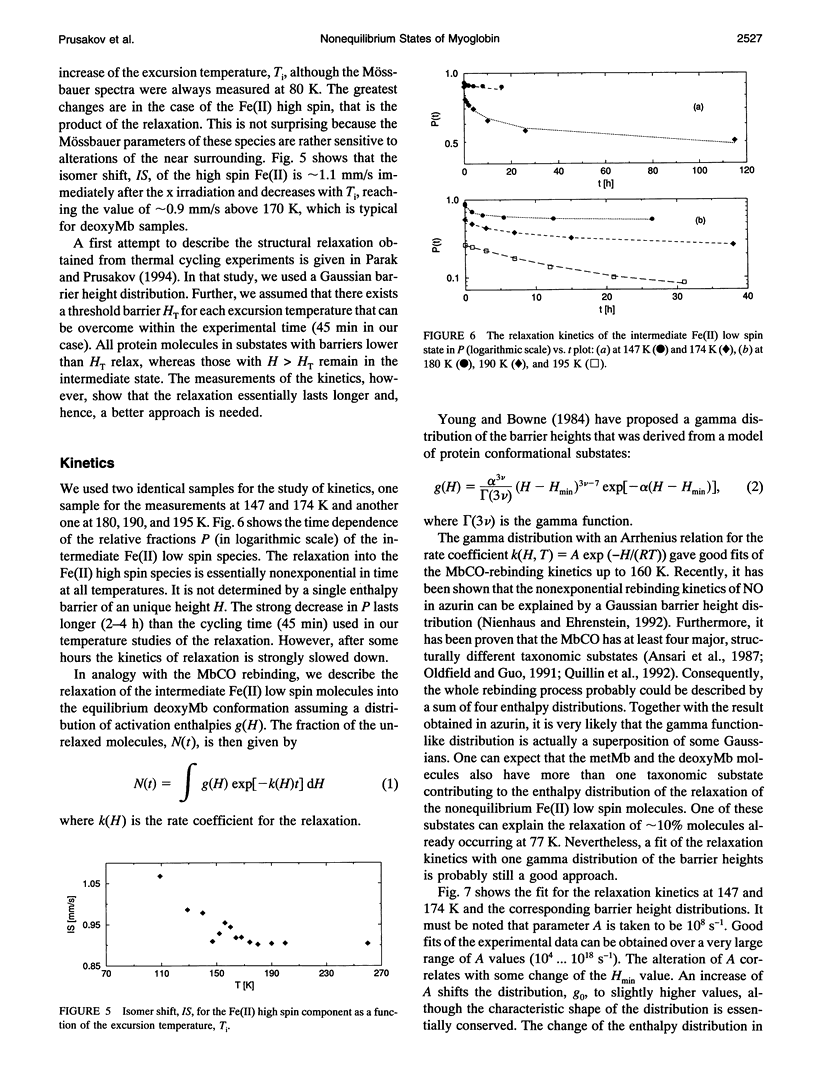
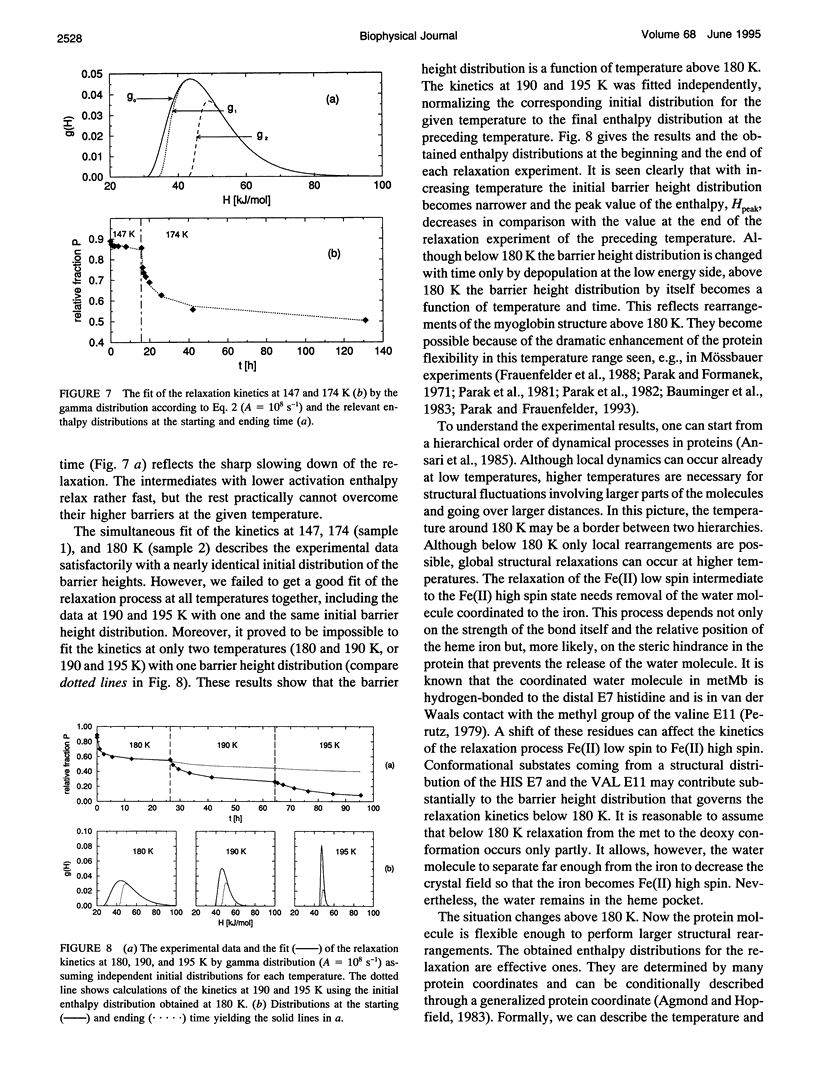
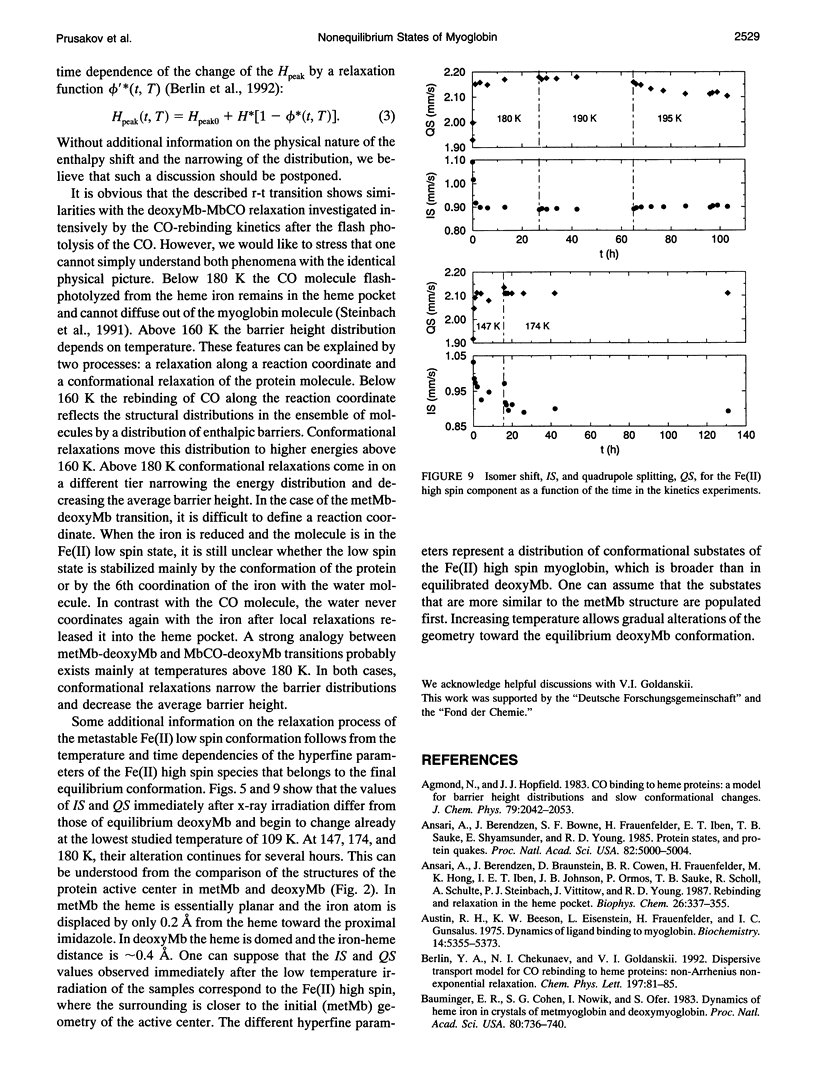
A frozen solution of 57Fe-enriched metmyoglobin was irradiated by x rays at 77 K. Mössbauer spectra showed a reduction of Fe(III) high spin by thermalized electrons and a production of a metastable Fe(II) low spin myoglobin complex with H2O at its sixth coordination site. The relaxation of the intermediate was investigated by Mössbauer spectroscopy as a function of temperature and time. The relaxation process starts above 140 K and is fully completed at approximately 200 K. At temperatures between 140 and 200 K, the relaxation lasts for hours and is nonexponential in time. Up to 180 K, the process can be described satisfactorily by a gamma distribution of activation enthalpies with an Arrhenius relation for the rate coefficient. The temperature and time dependence of the Mössbauer parameters indicates structural changes in the active center of the protein as early as 109 K that continue for several hours at higher temperatures. Above 180 K, structural rearrangements involving the whole protein molecule lead to a shift and narrowing of the barrier height distribution.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A., Berendzen J., Bowne S. F., Frauenfelder H., Iben I. E., Sauke T. B., Shyamsunder E., Young R. D. Protein states and proteinquakes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5000–5004. doi: 10.1073/pnas.82.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Johnson J. B., Ormos P., Sauke T. B. Rebinding and relaxation in the myoglobin pocket. Biophys Chem. 1987 May 9;26(2-3):337–355. doi: 10.1016/0301-4622(87)80034-0. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Bauminger E. R., Cohen S. G., Nowik I., Ofer S., Yariv J. Dynamics of heme iron in crystals of metmyoglobin and deoxymyoglobin. Proc Natl Acad Sci U S A. 1983 Feb;80(3):736–740. doi: 10.1073/pnas.80.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzarri A. R., Cannistraro S. Solvent modulation of the structural heterogeneity in FeIII myoglobin samples: a low temperature EPR investigation. Eur Biophys J. 1993;22(4):259–267. doi: 10.1007/BF00180260. [DOI] [PubMed] [Google Scholar]
- Doster W., Beece D., Bowne S. F., DiIorio E. E., Eisenstein L., Frauenfelder H., Reinisch L., Shyamsunder E., Winterhalter K. H., Yue K. T. Control and pH dependence of ligand binding to heme proteins. Biochemistry. 1982 Sep 28;21(20):4831–4839. doi: 10.1021/bi00263a001. [DOI] [PubMed] [Google Scholar]
- Ehrenstein D., Nienhaus G. U. Conformational substates in azurin. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9681–9685. doi: 10.1073/pnas.89.20.9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Köhler W, Friedrich J, Scheer H. Conformational barriers in low-temperature proteins and glasses. Phys Rev A Gen Phys. 1988 Jan 15;37(2):660–662. doi: 10.1103/physreva.37.660. [DOI] [PubMed] [Google Scholar]
- Nienhaus G. U., Mourant J. R., Frauenfelder H. Spectroscopic evidence for conformational relaxation in myoglobin. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2902–2906. doi: 10.1073/pnas.89.7.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parak F., Frolov E. N., Mössbauer R. L., Goldanskii V. I. Dynamics of metmyoglobin crystals investigated by nuclear gamma resonance absorption. J Mol Biol. 1981 Feb 5;145(4):825–833. doi: 10.1016/0022-2836(81)90317-x. [DOI] [PubMed] [Google Scholar]
- Parak F., Hartmann H., Aumann K. D., Reuscher H., Rennekamp G., Bartunik H., Steigemann W. Low temperature X-ray investigation of structural distributions in myoglobin. Eur Biophys J. 1987;15(4):237–249. doi: 10.1007/BF00577072. [DOI] [PubMed] [Google Scholar]
- Parak F., Knapp E. W., Kucheida D. Protein dynamics. Mössbauer spectroscopy on deoxymyoglobin crystals. J Mol Biol. 1982 Oct 15;161(1):177–194. doi: 10.1016/0022-2836(82)90285-6. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Regulation of oxygen affinity of hemoglobin: influence of structure of the globin on the heme iron. Annu Rev Biochem. 1979;48:327–386. doi: 10.1146/annurev.bi.48.070179.001551. [DOI] [PubMed] [Google Scholar]
- Steinbach P. J., Ansari A., Berendzen J., Braunstein D., Chu K., Cowen B. R., Ehrenstein D., Frauenfelder H., Johnson J. B., Lamb D. C. Ligand binding to heme proteins: connection between dynamics and function. Biochemistry. 1991 Apr 23;30(16):3988–4001. doi: 10.1021/bi00230a026. [DOI] [PubMed] [Google Scholar]
- Zollfrank J., Friedrich J., Parak F. Spectral hole burning study of protoporphyrin IX substituted myoglobin. Biophys J. 1992 Mar;61(3):716–724. doi: 10.1016/S0006-3495(92)81876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


