Abstract
Intracellular persistence of mycobacteria may result from an intricate balance between bacterial replication and signaling events leading to antimicrobial macrophage activities. Using human monocyte-derived macrophages, we investigated the relevance of mitogen-activated protein kinase activation for the growth control of Mycobacterium avium isolates differing in their abilities to multiply intracellularly. The highly replicative smooth transparent morphotype of M. avium strain 2151 induced significantly less p38 and extracellular signal-regulated kinases 1 and 2 (ERK1/2) phosphorylation than the smooth opaque morphotype of the same strain, which was gradually eliminated from macrophage cultures. Inhibition of the p38 pathway by highly specific inhibitors did not significantly affect mycobacterial replication within macrophages, regardless of the in vitro virulence of the M. avium strain. However, repression of the ERK1/2 pathway further enhanced intracellular growth of highly replicative M. avium strains, although it did not increase survival of the poorly replicating M. avium isolate. Inhibition of the ERK1/2 pathway resulted in decreased tumor necrosis alpha (TNF-α) secretion irrespective of the virulence of the M. avium isolate used for infection, revealing that TNF-α could have been only partially responsible for the control of intracellular M. avium growth. In conclusion, ERK1/2- and TNF-α-independent pathways are sufficient to limit intramacrophage growth of less-virulent M. avium strains, but early ERK1/2 activation in infected macrophages is critically involved in controlling the growth of highly replicative M. avium strains.
Macrophages play a central role in the first line of defense against pathogenic microorganisms because they are critically involved in the activation of both innate and acquired immune responses. Following phagocytosis, macrophages become activated to initiate defense mechanisms, e.g., production of nitric oxide and phagosome acidification, that ultimately lead to the degradation of many microbial species (33; reviewed in reference 19). Paradoxically, macrophages are also the key target cells of a variety of pathogens, e.g., mycobacteria, that have developed strategies to invade macrophages and replicate intracellularly.
Infections with mycobacteria, such as tuberculosis, are characterized by their chronic course. Both human and mouse studies have provided ample evidence that even in the face of an adequate immune response, mycobacteria like Mycobacterium tuberculosis and Mycobacterium avium are able to persist inside macrophages (13; reviewed in reference 20). Of interest, several strains and distinct morphotypes (smooth transparent, smooth opaque) of M. avium differ with respect to virulence and persistence in an in vivo infection model (25). One potential mechanism by which virulent mycobacterial strains, as opposed to avirulent strains, may achieve a state of long-term persistence is the modulation of signaling cascades leading to macrophage activation (reviewed in reference 23).
Diverse signaling cascades are involved in triggering cellular responses to pathogenic organisms (reviewed in reference 22). One essential branch of cell signaling in eucaryotic organisms is the ubiquitously expressed family of mitogen-activated protein (MAP) kinases (reviewed in reference 7). These serine/threonine kinases are critically involved in cell proliferation, differentiation, and cell death, as well as the inflammatory response (reviewed in reference 17). In mammals there are three subfamilies of MAP kinases that can be activated independently and simultaneously: p46 and p54 c-Jun-NH2-terminal kinases, p42 and p44 extracellular signal-regulated kinases 1 and 2 (ERK1/2), and p38 MAP kinase (reviewed in reference 5). MAP kinases mediate cellular responses to a variety of extracellular stimuli, such as physical stress (e.g., osmotic changes), inflammatory cytokines, growth factors, and bacterial components (e.g., lipopolysaccharide [LPS]) (reviewed in reference 18). Highly specific, cell-permeable inhibitors of MAP kinase activity have been useful tools in identifying some physiological functions of these signaling cascades in terms of infectious processes. By using these inhibitors, the life cycles of some viruses, e.g., human immunodeficiency virus, were determined to depend on ERK1/2 and p38 MAP kinase activity (29, 32, 34). ERK1/2 activity was also shown to be critically involved in invasion of the facultative intracellular bacterium Listeria monocytogenes, but not Salmonella enterica serovar Typhimurium (31). Even growth of some tumors in vivo was successfully blocked by MAP kinase inhibitors (28).
The activation of MAP kinase signaling cascades by mycobacterial components (6, 15) as well as viable M. avium (26) has only recently been described. However, the functional relevance of MAP kinase activity with respect to uptake and intracellular persistence of mycobacteria has remained unexplored. In particular, it is unknown whether there is a direct correlation between the magnitude of MAP kinase activation and the magnitude of intracellular replication of different M. avium strains or morphotypes. In this study, we investigated whether highly specific MAP kinase inhibitors would interfere with intramacrophage growth and cytokine induction of M. avium strains differing in their in vitro replication rates.
MATERIALS AND METHODS
Bacteria.
M. avium strains 2151 (morphotypes smooth transparent and smooth opaque) and SE01 were originally isolated from AIDS patients (3, 13). Mycobacterial strains were grown in Middlebrook 7H9 medium (Difco, Detroit, Mich.) containing 10% OADC (oleic acid, albumin, dextrose, catalase; Becton Dickinson) and 0.05% Tween 80 (Sigma, Deisenhofen, Germany) until mid-log phase. Absence of contaminating microorganisms was verified by plating culture material on brain heart infusion agar (Difco) and Ziehl-Neelsen staining. The suspension was frozen in aliquots at −70°C until use. For calculation of CFU, aliquots were serially diluted in sterile, distilled water containing 0.05% Tween 80 and plated on 7H10 agar containing 0.075% pyruvate (Sigma). After incubation for 3 weeks at 37°C, CFU were calculated. For infection, bacterial aliquots were thawed and centrifuged for 10 min at 835 × g. Bacteria were resuspended in phosphate-buffered saline and sonicated (3 to 5 min, 35 kHz; Bandelin, Berlin, Germany) to disrupt aggregates. Bacterial LPS of S. enterica serotype Friedenau H909 was kindly provided by H. Brade (Research Center Borstel, Borstel, Germany).
Inhibitors.
PD98059 and SB203580 were purchased from Calbiochem (Schwalbach, Germany) and used at concentrations of 3 or 10 μM. Dimethyl sulfoxide (DMSO; Sigma) was added to cultures at 0.1% (vol/vol) as solvent control.
Isolation and cultivation of human monocyte-derived macrophages.
Mononuclear cells were isolated from the peripheral blood of healthy volunteers by density gradient centrifugation (4). Lymphocytes and monocytes were separated by counterflow elutriation as previously described (12). Highly purified (consistently >95%) monocytes were cultivated for 7 days in Teflon culture bags (CellGenix, Freiburg, Germany) in RPMI 1640 (Biochrom, Berlin, Germany) in the presence of 2% (vol/vol) heat-inactivated human AB serum, 2 ng of human macrophage colony-stimulating factor (R&D Systems, Wiesbaden, Germany) per ml, 2 mmol of l-glutamine (Biochrom) per liter, 100 U of penicillin G per ml, and 100 μg of streptomycin/ml (both from Biochrom). Cell viability was determined by trypan blue staining. Macrophages were phenotyped by cell surface marker expression profile (CD14, macrophage mannose receptor, carboxypeptidase M, and HLA-DR) as described previously (26). Macrophages were cultivated in 24-well flat-bottom microtiter plates (Nunc, Roskilde, Denmark) in RPMI 1640 containing 10% (vol/vol) heat-inactivated fetal calf serum (Biochrom) and 2 mmol of l-glutamine/liter. Cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2.
In vitro infection.
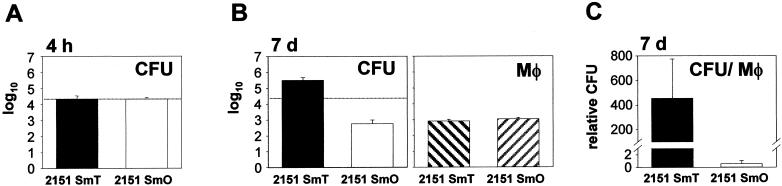
A total of 2.5 × 105 human macrophages (at a concentration of 5 × 105 per ml) were inoculated with 1.25 × 106 CFU of M. avium 2151 SmT or 2151 SmO per well, and cultures were incubated in the presence or absence of MAP kinase inhibitors. After 4 h, cells were washed vigorously with warm Hank's balanced salt solution to remove extracellular bacteria. To determine bacterial uptake, macrophages were lysed by addition of 0.1% saponin. Lysates were serially diluted and plated on agar (see above). For the investigation of bacterial growth, parallel cultures were maintained for 7 days in 500 μl of medium (at day three, 500 μl of fresh medium was added). Subsequently, cells were washed, lysed, diluted, and plated for CFU determination as described above. In several experiments donor-dependent detachment of macrophages was noted to a varying degree after 7 days of infection. To take into account potential differences in the survival rates of macrophages infected with mycobacterial strains of different virulences under different assay conditions, the actual amount of mycobacteria inside macrophages still present at the end of culture was determined. To this end, adherent macrophages were quantified at the end point of infection as follows: in each well, two identical areas (each 2 mm2 in size) equidistant from the center of the well (1 mm above and 1 mm below) were defined using a superimposed grid. Within any one individual well, these two indicator areas showed comparable densities and distributions of macrophages. Using a digital camera (Visicam 1300; Vitron Systems GmbH, Munich, Germany) attached to a microscope (DMIRB, Leica Microsystems AG, Wetzlar, Germany) and imaging software (MetaMorph 3.0; Universal Imaging, West Chester, Pa.), the indicator areas were photographed and macrophages present in these areas were counted manually on printouts. Subsequently, CFU counts per well were divided by the mean number of macrophages in these indicator areas. This index allows direct comparison of mycobacteria surviving within macrophages of different wells and defines the relative CFU, shown below in Fig. 1 and 3.
FIG. 1.
Uptake and intracellular replication of M. avium 2151 SmT and SmO in human macrophages. Human monocyte-derived macrophages were infected with the smooth transparent (SmT) or the smooth opaque (SmO) morphotype of M. avium strain 2151. (A) CFU counts in lysed macrophages were determined 4 h after infection. (B) Intracellular mycobacterial replication was determined by CFU counts after 7 days and compared to initial intracellular bacterial numbers (dotted line). Macrophage (Mφ) numbers in indicator areas were determined. (C) To meaningfully compare actual intracellular mycobacterial replication rates within macrophages, CFU were correlated to macrophage numbers at day 7. Relative CFU were calculated by dividing CFU per well by the number of macrophages in indicator areas (as described in Materials and Methods). Means of duplicates ± SD of one typical experiment out of six (six different donors) are shown.
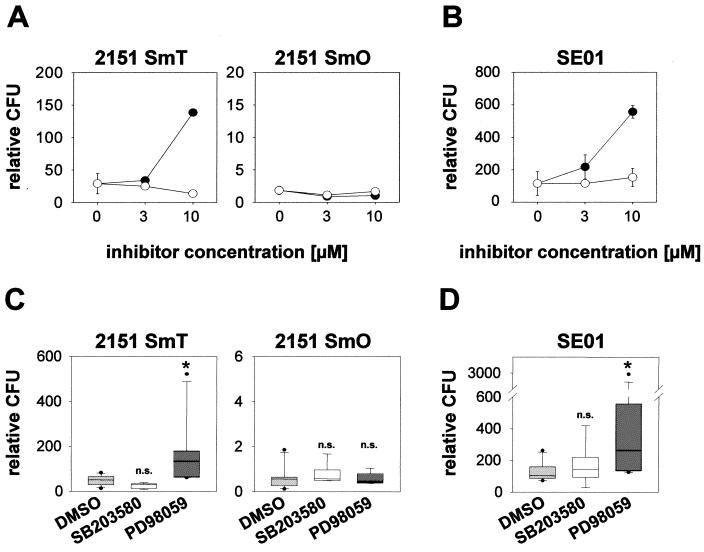
FIG. 3.
PD98059, but not SB203580, enhances intracellular mycobacterial growth. Cultures of human monocyte-derived macrophages were preincubated with 3 or 10 μM PD98059 (•) or SB203580 (○) or 0.1% (vol/vol) DMSO as solvent control for 60 min. Macrophages were infected with M. avium 2151 smooth transparent (SmT) or smooth opaque (SmO) (A) or M. avium strain SE01 (B). The number of intracellular viable bacteria correlated to macrophage numbers (relative CFU) was determined 7 days postinfection. Values represent means of duplicates ± SD of one representative experiment out of six. (C and D) Results obtained from different donors. Macrophages were preincubated with 10 μM MAP kinase inhibitor or 0.1% (vol/vol) DMSO. Cells were infected with 2151 SmT or 2151 SmO (C) or with SE01 (D). For each donor, CFU counts 7 days postinfection were correlated to macrophage numbers and are shown as relative CFU. The boxes indicate the 25th and 75th percentiles. Whiskers above and below the boxes indicate the 90th and 10th percentiles. Medians are marked. All outlying points are graphed. Data shown were obtained from six independent experiments (six different donors) performed in duplicate. *, P < 0.05; n.s., not significant.
Determination of MAP kinase phosphorylation.
A total of 4 × 105 human macrophages (at a concentration of 8 × 105 per ml) were stimulated with 2 × 106 CFU of M. avium or 10 ng of LPS/ml as a control for 30 min. Cultures were lysed by addition of 100 μl of 2× sample buffer (125 mM Tris, pH 6.8; 4% sodium dodecyl sulfate [SDS]; 20% glycerol; 100 mM dithiothreitol; 0.05% bromphenol blue). Samples were loaded onto an SDS-12% polyacrylamide gel and run at 100 mA for 1 h. Proteins were transferred onto a nitrocellulose membrane (Sartorius, Göttingen, Germany) by wet blotting at 1.5 mA/cm2 (4°C) for 1.5 h. After blocking with 5% (wt/vol) nonfat dry milk (Gluecksklee, Nestlé, Frankfurt, Germany) in Tris-buffered saline containing 0.1% Tween 20 (Sigma) (TTBS), membranes were incubated with the primary antibody (p38, phospho-p38, ERK1/2, and phospho-ERK1/2; New England Biolabs, Schwalbach, Germany) overnight at 4°C. Blots were washed three times with TTBS and incubated for 1 h with the horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Dianova, Hamburg, Germany). Membranes were developed using a chemiluminescence assay (ECL; Pharmacia-Amersham, Freiburg, Germany) and subsequently exposed to chemiluminescence film (Pharmacia-Amersham).
Detection of cytokine release.
A total of 4 × 105 human macrophages (at a concentration of 8 × 105 per ml) were stimulated with 2 × 106 CFU of M. avium or 10 ng of LPS/ml as a control. Culture supernatants of stimulated macrophages were harvested at the time points indicated and stored at −20°C until analysis. Sandwich enzyme-linked immunosorbent assays (ELISAs) were used for detecting tumor necrosis factor alpha (TNF-α; H. Gallati, Intex, Muttenz, Switzerland) (11) and interleukin-10 (IL-10) (R&D Systems) in culture supernatants. Assays were performed as recommended by the manufacturers.
Statistical analysis.
The effects of p38 and ERK inhibition on intramacrophage mycobacterial replication and cytokine secretion were analyzed in infection experiments with six and three different donors, respectively. For statistical analysis, data from independent infection experiments were log transformed prior to analysis. Two-factorial analysis of variance with the donor as blocking factor and subgroup analysis were performed by using a one-sided t test (JMP 4.0; SAS Institute, Cary, N.C.). The α-error was corrected according to the Bonferroni-Holm procedure. Cytokine concentrations under different culture conditions were correlated to concentrations in control cultures (normalized to 100%). Data obtained from independent experiments were compared by using a t test for multiple comparisons and are represented as the mean ± standard deviation (SD).
RESULTS
M. avium 2151 SmT and SmO show different growth kinetics in human macrophages in vitro.
Human macrophages were infected with either the smooth transparent (SmT) or the smooth opaque (SmO) morphotype of M. avium 2151. After 4 h, both morphotypes were phagocytosed at comparable numbers (Fig. 1A) with a multiplicity of infection averaging 1 mycobacterium per 12 to 14 macrophages. Mycobacterial growth in human macrophages 7 days after infection is shown in Fig. 1B. The SmT morphotype replicated extensively compared to the SmO morphotype, which was gradually eliminated during infection. In the absence of macrophages, both morphotypes replicated to a comparable extent in cell culture medium (data not shown). Although the two morphotypes differentially persisted in infected cultures, macrophage numbers were identical 7 days postinfection (Fig. 1B). When depicted as CFU related to macrophage number (relative CFU, as described in Materials and Methods), a 774-fold increase in CFU of SmT above that of SmO was observed (Fig. 1C).
M. avium-induced MAP kinase activation is inversely correlated to in vitro virulence of the M. avium morphotypes.
In order to investigate whether the divergent intracellular replication rates of the two morphotypes were related to differential host cell activation, M. avium was added to human macrophage cultures and phosphorylation of the p38 and ERK1/2 (p44/p42) MAP kinases was analyzed. In macrophages infected with the highly replicative SmT morphotype, only a weak phosphorylation of both p38 and ERK1/2 was observed. In contrast, a markedly stronger signal of p38 and ERK1/2 phosphorylation was detected after addition of the poorly persisting SmO morphotype to the macrophages (Fig. 2). Both M. avium morphotypes induced p38 and ERK1/2 phosphorylation with identical kinetics, starting with detectable phosphorylation as early as 15 min (data not shown) and with a maximum at 30 to 45 min. Thus, the magnitude of MAP kinase phosphorylation induced by M. avium was inversely correlated to intracellular mycobacterial replication.
FIG. 2.
Differential phosphorylation of MAP kinases by M. avium 2151 SmT and SmO. Human monocyte-derived macrophages were incubated with either the smooth transparent (SmT) or smooth opaque (SmO) morphotype of M. avium strain 2151 or LPS for 30 min. Cells were lysed, and aliquots of cell lysates were separated by SDS-polyacrylamide gel electrophoresis and immunoblotted onto nitrocellulose membranes. The membranes were incubated with specific anti-phospho-ERK1/2 and anti-phospho-p38 antibodies and then incubated with a peroxidase-coupled secondary antibody. Visualization was performed by enhanced chemiluminescence. As controls, the amounts of total ERK1/2 and p38 were detected in the same lysates. One representative experiment out of three independent experiments (three different donors) is shown.
ERK1/2, but not p38, activity is involved in control of intracellular M. avium replication in human macrophages.
In order to investigate whether MAP kinase activity was directly involved in mycobacterial survival, infection experiments were performed in the presence or absence of highly specific pharmacologic inhibitors of MAP kinase signaling cascades. PD98059 is a well-established inhibitor of the ERK1/2 pathway that acts by repressing activation of MEK-1, the kinase upstream of p42 and p44. The pyridinyl imidazole SB203580 specifically inhibits p38 activity by binding to the ATP-binding site and inhibiting enzyme activity but not p38 phosphorylation (reviewed in reference 8 and references therein).
The presence of MAP kinase inhibitors did not significantly affect mycobacterial uptake into macrophages 4 h postinfection compared to cultures with no additive or with DMSO as solvent control (data not shown). After 7 days, CFU of the highly replicative SmT morphotype had substantially increased in cultures containing PD98059 (10 μM), whereas the presence of SB203580 did not have any effect even at concentrations up to 10 μM. The gradual elimination of the SmO morphotype from macrophages was unaffected after coincubation with either PD98059 or SB203580 (Fig. 3A). In order to validate the observed growth-enhancing effect of PD98059 on the highly replicative variant 2151 SmT, parallel cultures were infected with another strain of M. avium (SE01) which also showed extensive growth in macrophages. Again, the presence of PD98059 led to an increase in mycobacterial CFU relative to macrophage numbers, while SB203580 did not alter intracellular growth of this strain (Fig. 3B). DMSO alone had no influence on mycobacterial growth. In addition, a direct effect of PD98059 and SB203580 on mycobacterial growth was excluded by cultivation of mycobacteria in the presence of MAP kinase inhibitors but in the absence of macrophages (data not shown).
The same set of experiments was performed independently for six different donors. Although there was substantial donor-dependent variation with regard to the extent of mycobacterial proliferation within macrophages, the presence of PD98059 led to a significant increase in colony counts of the highly proliferative strains 2151 SmT (Fig. 3C) and SE01 (Fig. 3D). These results were confirmed by using a second, more potent MEK-1 inhibitor, U0126 (data not shown). In contrast, the presence of PD98059 did not significantly alter CFU counts of the SmO morphotype, and mycobacterial growth was repressed in all cases (Fig. 3C). The presence of SB203580 consistently had no significant effect on intramacrophage replication rates of any of the M. avium strains tested (Fig. 3C and D).
Therefore, activation of the ERK1/2 pathway seems to be critically involved in controlling intracellular mycobacterial replication of highly replicative M. avium strains.
Cytokines are only partially involved in control of intramacrophage growth of M. avium.
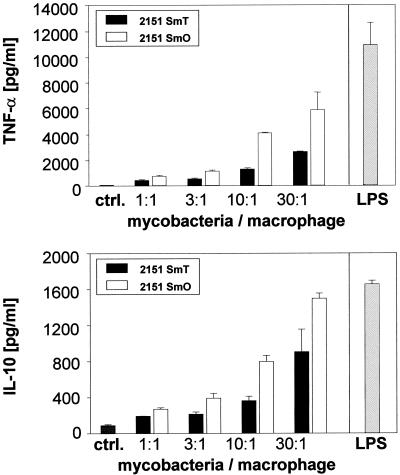
To gain further insight into the mechanisms by which inhibition of the ERK1/2 pathway might exert its intramacrophage growth-promoting effect on certain M. avium strains, the magnitude of cytokine secretion induced by M. avium strains differing in their virulence was analyzed in the presence and absence of MAP kinase inhibitors. To this end, human macrophages were infected with M. avium 2151 SmT or SmO and the levels of the macrophage-activating cytokine TNF-α and the macrophage-deactivating cytokine IL-10 into the supernatant were analyzed. Both M. avium morphotypes induced the release of TNF-α and IL-10 in a dose-dependent manner (Fig. 4). The highly replicative SmT morphotype, which led to only a weak MAP kinase activation, induced the release of significantly smaller amounts (up to three times less than SmO) of TNF-α and IL-10. In contrast, the SmO morphotype, a strong activator of MAP kinases, led to a strong induction of cytokine release. Therefore, M. avium-induced TNF-α and IL-10 release reflected the extent of MAP kinase activation by morphotypes differing in their intracellular replication rates in vitro.
FIG. 4.
TNF-α and IL-10 release in cultures of human macrophages infected with M. avium strain 2151 SmT or SmO. Human monocyte-derived macrophages were incubated with increasing numbers of M. avium 2151 smooth transparent (SmT) or smooth opaque (SmO) or LPS as positive control. Supernatants were harvested after 18 h (TNF-α) and additionally from the same cultures 44 h (IL-10) postinfection. Cytokine concentrations were measured by ELISA. Means of duplicates (± SD) of one representative experiment out of three (three different donors) are shown.
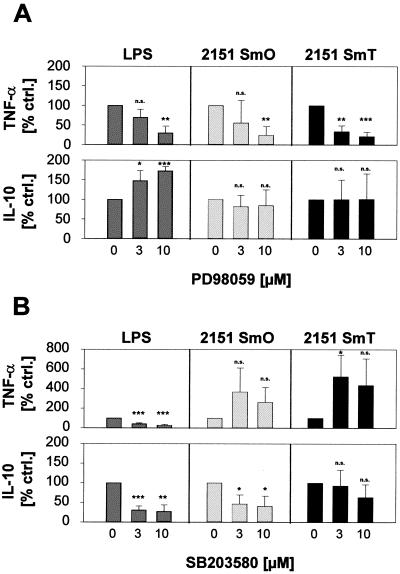
Since inhibition of p38 and ERK1/2 activities had different effects on the intracellular replication rates of the M. avium strains examined, it appeared possible that inhibition of p38 and ERK1/2 activities in macrophages infected with these strains would have strain- or virulence-dependent effects on TNF-α and IL-10 secretion. Our data (Fig. 5) clearly show, however, that the effect of MAP kinase inhibition on cytokine expression was not virulence specific. In fact, a differential effect of MAP kinase inhibition on TNF-α and IL-10 secretion, previously demonstrated by our laboratory for M. avium strain SE01 (26), was seen for both morphotypes of strain 2151 and was, therefore, independent of the virulence of the M. avium isolate. TNF-α release was significantly reduced by PD98059 in a dose-dependent manner after stimulation with either M. avium strain. In contrast, IL-10 formation was not inhibited in the presence of PD98059 (Fig. 5A). Inhibition of p38 activity by cocultivation with SB203580 did not reduce TNF-α formation in macrophages induced by either morphotype. Although statistically not significant, M. avium-induced TNF-α release was usually enhanced. Strain 2151 SmT always induced little IL-10 formation (Fig. 4). Usage of SB203580 further reduced IL-10 release, although effects were not found to be statistically significant because all measured IL-10 amounts were near the lower detection limit of the ELISA (Fig. 5B). The highly activating smooth opaque morphotype induced the formation of higher amounts of IL-10. In this case, IL-10 release was significantly reduced in the presence of SB203580 (Fig. 5B).
FIG. 5.
PD98059 reduces M. avium-induced TNF-α formation; SB203580 reduces M. avium-induced IL-10 release. Human monocyte-derived macrophages were preincubated with medium containing 3 or 10 μM PD98059 (A) or SB203580 (B), or 0.1% (vol/vol) DMSO as solvent control for 60 min. Cultures were incubated with M. avium 2151 smooth transparent (SmT) or smooth opaque (SmO) or LPS as a positive control. Supernatants were harvested after 18 h (TNF-α) or 44 h (IL-10), and cytokine concentrations were determined by ELISA. To compare results from different experiments showing substantial donor-dependent variation of cytokine concentrations, secreted amounts of TNF-α and IL-10 are shown as percent of control cultures stimulated in the presence of DMSO (normalized to 100%). Data shown are the mean ± SD of three independent experiments (three different donors) performed in duplicate. *, P < 0.05; **, P < 0.005; ***, P < 0.0005; n.s., not significant.
DISCUSSION
The aim of this study was to analyze whether the extent of MAP kinase signaling induced by various M. avium strains and morphotypes would be a determinant for their subsequent intracellular survival. We found that M. avium-induced activation of ERK1/2 and p38 MAP kinases was inversely correlated to the virulence of the infecting mycobacterial variant in vitro. Inhibition of ERK1/2 activation enhanced intracellular multiplication of highly replicative strains, whereas p38 activation was not involved in controlling intracellular growth or survival of either high-level or low-level replicating variants of M. avium. However, the regulation of M. avium-induced cytokine release was independent of mycobacterial virulence. We found ERK1/2 activation essential for TNF-α formation, whereas p38 activity, but not the ERK1/2 pathway, was involved in IL-10 release.
Infection with M. avium, a facultative intracellular opportunistic pathogen in humans, is a well-studied model for mycobacterial infections. A major phenotypic characteristic of M. avium isolates is their ability to appear in different colony morphotypes: smooth (either transparent or opaque) or rough (reviewed in reference 14). Although morphotypes of the same strain are very closely related, previous studies demonstrated that the smooth transparent variant is usually better able to survive and grow intracellularly than the opaque morphotype (10, 21). With regard to the M. avium morphotypes used in this study (2151 SmT and SmO), this pattern has been confirmed both in murine macrophages and in a mouse model of infection (1, 2). Our results extend these findings to human monocyte-derived macrophages. This is not trivial, because in the case of the M. tuberculosis strain H37Rv and its avirulent variant H37Ra, growth patterns in mice and murine macrophages are not reflected by their in vitro growth characteristics in human macrophages (24). Why different morphotypes of M. avium either possess or lack the capacity for intracellular persistence is entirely unknown, but the responsible mechanisms likely involve modulation of intracellular signaling pathways (reviewed in reference 23).
Focusing on MAP kinase signaling events, we observed activation of both ERK1/2 and p38 in response to the two closely related morphotypes of M. avium 2151. Since the magnitude of MAP kinase activation was inversely correlated to the intramacrophage replication potential of these strains, we hypothesized that ERK1/2 and p38 signaling might indeed play a direct role in controlling the growth of these strains.
A variety of commercially available compounds have proven to be powerful tools for functional analysis of particular cell signaling elements (8). The highly specific inhibitors PD98059 and SB203580 have previously been used to demonstrate involvement of ERK1/2 and p38 MAP kinase pathways in uptake and persistence of pathogenic viruses and bacteria (16, 29).
Regarding intracellular mycobacterial replication, inhibition of the ERK1/2 pathway led to an increased growth of M. avium 2151 SmT (and also of another, highly replicative strain, SE01) within human macrophages. This growth-enhancing effect was, however, not observed for the related SmO morphotype of the same strain. These data indicate that replication of some mycobacterial strains, such as M. avium 2151 SmO, can easily be controlled by macrophages, even in the presence of drugs interfering with essential signaling pathways. In this respect, it is interesting that ERK1/2 activity also seems not to be involved in controlling the replication of another facultative intracellular bacterium, L. monocytogenes (16). In contrast, other mycobacterial variants, like M. avium 2151 SmT and SE01, are more resistant to growth control by the macrophage. In macrophages infected with these strains, additional repression of the ERK1/2 pathway might unbalance innate defense mechanisms to an extent more favorable to mycobacterial replication. Notably, inhibition of p38 activity in human macrophages did not significantly affect intracellular replication of any of the M. avium variants used. From these results, we conclude that p38 activity is not critically involved in the control of mycobacterial growth in infected macrophages in vitro. Parallel infection studies performed in bone marrow-derived macrophages of C57BL/6 mice (A. Blumenthal et al., unpublished observations) corroborate our data and show that our results are not restricted to the human system.
One possible mechanism by which the magnitude of MAP kinase activity may influence intracellular mycobacterial replication could be the induction of cytokines. With regard to TNF-α, several previous studies have shown an inverse correlation between the extent of TNF-α release in reaction to M. avium and the virulence of the infecting strain or morphotype. Therefore, the hypothesis was put forth that certain M. avium isolates are more virulent because they induce only little TNF-α in vitro (10, 27). With respect to the two morphotypes of M. avium 2151, our data confirm this hypothesis and, in addition, provide a plausible explanation at the signal transduction level. Thus, the virulent morphotype induced less MAP kinase phosphorylation than the avirulent morphotype. Virulence may therefore simply be a reflection of an intrinsic inability of certain mycobacterial strains to stimulate macrophages. However, it is intriguing to speculate that virulence may also reside in the capacity of some mycobacterial strains to inhibit early signal transduction events, such as MAP kinase phosphorylation.
PD98059 and SB203580 were used to analyze whether MAP kinase activation would affect M. avium-induced cytokine release in a virulence-dependent way. As shown, independent of the morphotype used, TNF-α production was critically dependent on the activation of the ERK1/2 pathway, whereas p38 activity, and not ERK1/2, was involved in IL-10 release, which corroborates earlier studies using another well-replicating M. avium strain, SE01 (26).
We suggest that differential regulation of pro- and antiinflammatory mediators at the level of MAP kinase activity may represent a general macrophage response to M. avium (independent of the strain or morphotype) and, possibly, to other virulent and avirulent mycobacterial species.
Previous studies described contradictory effects of neutralization of endogenous IL-10 in vitro (9, 30). In our system, inhibition of endogenous IL-10 by SB203580 was not accompanied by enhanced growth of any M. avium strain tested. We conclude that IL-10 release by infected macrophages may not be critical in intracellular growth control of M. avium. Inhibition of the ERK1/2 signaling cascade in M. avium 2151 SmT- or SE01-infected macrophages was followed by diminished TNF-α release and enhanced intracellular mycobacterial replication. An important role for TNF-α in the control of M. avium replication in vitro has previously been demonstrated (1). Furney et al. (10) showed a significant inhibitory effect of TNF-α on intracellular replication only for extensively replicating M. avium variants, whereas the effect on low-level-replicating mycobacteria was minimal. Thus, early inhibition of TNF-α release may be one of the mechanisms by which PD98059 promotes intramacrophage growth of some highly replicative strains of M. avium.
A number of arguments, however, strengthen the view that control of M. avium replication insides macrophages involves more than stimulation by TNF-α. First, infection of macrophages with M. avium SE01 induces TNF-α levels comparable to those achieved by the easily controlled 2151 SmO (26; also data not shown). Yet, SE01 is a highly replicative M. avium strain and, in this respect, quite similar to 2151 SmT, which induces only low amounts of TNF-α. Second, inhibition of the ERK1/2 pathway markedly reduced TNF-α secretion induced by 2151 SmO, yet intracellular survival rates stayed low for this isolate.
Since the growth-enhancing effect of ERK1/2 pathway inhibition cannot be entirely explained by modulation of TNF-α levels, it would appear that M. avium-induced activation of the ERK1/2 signaling cascade is involved in other, TNF-α-independent cellular processes that ultimately lead to restriction of mycobacterial growth. Alternatively, it is entirely possible that M. avium strains simply differ in their susceptibility to TNF-α-induced bacteriostatic mechanisms and that, for some strains, higher TNF-α levels are required to achieve growth inhibition than for others.
Our present study demonstrates that, for M. avium infection, the relationship between macrophage activation (as reflected by MAP kinase phosphorylation), TNF-α and IL-10 secretion, and bacteriostasis is more complex than previously thought. We have identified ERK1/2 signaling as a critical pathway for growth restriction of some M. avium strains. We further uncovered that ERK1/2-independent mechanisms, as well as TNF-α-independent mechanisms, must exist to curtail the growth of other M. avium variants. It would appear that novel strategies for antimycobacterial immunotherapy will require a more thorough understanding of the subtleties of the host-pathogen relationship.
Acknowledgments
We thank Stefan Uhlig for helpful discussions. We gratefully acknowledge the expert technical assistance of R. Bergmann, E. Kaltenhäuser, S. Kröger, and J. Suwinski.
This work was supported in part by the Deutsche Forschungsgemeinschaft, SFB 415, project C7.
Editor: J. D. Clements
REFERENCES
- 1.Appelberg, R., and I. M. Orme. 1993. Effector mechanisms involved in cytokine-mediated bacteriostasis of Mycobacterium avium infections in murine macrophages. Immunology 80:352-359. [PMC free article] [PubMed] [Google Scholar]
- 2.Appelberg, R., A. Sarmento, and A. G. Castro. 1995. Tumour necrosis factor-alpha (TNF-alpha) in the host resistance to mycobacteria of distinct virulence. Clin. Exp. Immunol. 101:308-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belisle, J. T., K. Klaczkiewicz, P. J. Brennan, W. R. J. Jacobs, and J. M. Inamine. 1993. Rough morphological variants of Mycobacterium avium. Characterization of genomic deletions resulting in the loss of glycopeptidolipid expression. J. Biol. Chem. 268:10517-10523. [PubMed] [Google Scholar]
- 4.Bøyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. Suppl. 97:77-89. [PubMed] [Google Scholar]
- 5.Cano, E., and L. C. Mahadevan. 1995. Parallel signal processing among mammalian MAPKs. Trends Biochem. Sci. 20:117-122. [DOI] [PubMed] [Google Scholar]
- 6.Chan, E. D., K. R. Morris, J. T. Belisle, P. Hill, L. K. Remigio, P. J. Brennan, and D. W. Riches. 2001. Induction of inducible nitric oxide synthase-NO by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-κB signaling pathways. Infect. Immun. 69:2001-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 8.Davies, S. P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denis, M., and E. Ghadirian. 1993. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J. Immunol. 151:5425-5430. [PubMed] [Google Scholar]
- 10.Furney, S. K., P. S. Skinner, A. D. Roberts, R. Appelberg, and I. M. Orme. 1992. Capacity of Mycobacterium avium isolates to grow well or poorly in murine macrophages resides in their ability to induce secretion of tumor necrosis factor. Infect. Immun. 60:4410-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallati, H., and I. Pracht. 1985. Horseradish peroxidase: kinetic studies and optimization of peroxidase activity determination using the substrates H2O2 and 3,3′,5,5′-tetramethylbenzidine. J. Clin. Chem. Clin. Biochem. 23:453-460. [PubMed] [Google Scholar]
- 12.Grage-Griebenow, E., D. Lorenzen, R. Fetting, H.-D. Flad, and M. Ernst. 1993. Phenotypical and functional characterization of Fc gamma receptor I (CD64)-negative monocytes, a minor human monocyte subpopulation with high accessory and antiviral activity. Eur. J. Immunol. 23:3126-3135. [DOI] [PubMed] [Google Scholar]
- 13.Hänsch, H. C., D. A. Smith, M. E. Mielke, H. Hahn, G. J. Bancroft, and S. Ehlers. 1996. Mechanisms of granuloma formation in murine Mycobacterium avium infection: the contribution of CD4+ T cells. Int. Immunol. 8:1299-1310. [DOI] [PubMed] [Google Scholar]
- 14.Inderlied, C. B., C. A. Kemper, and L. E. Bermudez. 1993. The Mycobacterium avium complex. Clin. Microbiol. Rev. 6:266-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones, B. W., T. K. Means, K. A. Heldwein, M. A. Keen, P. J. Hill, J. T. Belisle, and M. J. Fenton. 2001. Different Toll-like receptor agonists induce distinct macrophage responses. J. Leukoc. Biol. 69:1036-1044. [PubMed] [Google Scholar]
- 16.Kugler, S., S. Schuller, and W. Goebel. 1997. Involvement of MAP-kinases and -phosphatases in uptake and intracellular replication of Listeria monocytogenes in J774 macrophage cells. FEMS Microbiol. Lett. 157:131-136. [DOI] [PubMed] [Google Scholar]
- 17.Kyriakis, J. M., and J. Avruch. 1996. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J. Biol. Chem. 271:24313-24316. [DOI] [PubMed] [Google Scholar]
- 18.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 19.MacMicking, J., Q. W. Xie, and C. Nathan. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15:323-350. [DOI] [PubMed] [Google Scholar]
- 20.Manabe, Y. C., and W. R. Bishai. 2000. Latent Mycobacterium tuberculosis—persistence, patience, and winning by waiting. Nat. Med. 6:1327-1329. [DOI] [PubMed] [Google Scholar]
- 21.Meylan, P. R., D. D. Richman, and R. S. Kornbluth. 1990. Characterization and growth in human macrophages of Mycobacterium avium complex strains isolated from the blood of patients with acquired immunodeficiency syndrome. Infect. Immun. 58:2564-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muzio, M., G. Natoli, S. Saccani, M. Levrero, and A. Mantovani. 1998. The human Toll signaling pathway: divergence of nuclear factor κB and JNK/SAPK activation upstream of tumor necrosis factor receptor-associated factor 6 (TRAF6). J. Exp. Med. 187:2097-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nandan, D., K. L. Knutson, R. Lo, and N. E. Reiner. 2000. Exploitation of host cell signaling machinery: activation of macrophage phosphotyrosine phosphatases as a novel mechanism of molecular microbial pathogenesis. J. Leukoc. Biol. 67:464-470. [DOI] [PubMed] [Google Scholar]
- 24.Paul, S., P. Laochumroonvorapong, and G. Kaplan. 1996. Comparable growth of virulent and avirulent Mycobacterium tuberculosis in human macrophages in vitro. J. Infect. Dis. 174:105-112. [DOI] [PubMed] [Google Scholar]
- 25.Pedrosa, J., M. Florido, Z. M. Kunze, A. G. Castro, F. Portaels, J. McFadden, M. T. Silva, and R. Appelberg. 1994. Characterization of the virulence of Mycobacterium avium complex (MAC) isolates in mice. Clin. Exp. Immunol. 98:210-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiling, N., A. Blumenthal, H. D. Flad, M. Ernst, and S. Ehlers. 2001. Mycobacteria-induced TNF-alpha and IL-10 formation by human macrophages is differentially regulated at the level of mitogen-activated protein kinase activity. J. Immunol. 167:3339-3345. [DOI] [PubMed] [Google Scholar]
- 27.Sarmento, A. M., and R. Appelberg. 1995. Relationship between virulence of Mycobacterium avium strains and induction of tumor necrosis factor alpha production in infected mice and in in vitro-cultured mouse macrophages. Infect. Immun. 63:3759-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sebolt-Leopold, J. S., D. T. Dudley, R. Herrera, K. Van Becelaere, A. Wiland, R. C. Gowan, H. Tecle, S. D. Barrett, A. Bridges, S. Przybranowski, W. R. Leopold, and A. R. Saltiel. 1999. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat. Med. 5:810-816. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro, L., K. A. Heidenreich, M. K. Meintzer, and C. A. Dinarello. 1998. Role of p38 mitogen-activated protein kinase in HIV type 1 production in vitro. Proc. Natl. Acad. Sci. USA 95:7422-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiratsuchi, H., B. Hamilton, Z. Toossi, and J. J. Ellner. 1996. Evidence against a role for interleukin-10 in the regulation of growth of Mycobacterium avium in human monocytes. J. Infect. Dis. 173:410-417. [DOI] [PubMed] [Google Scholar]
- 31.Tang, P., C. L. Sutherland, M. R. Gold, and B. B. Finlay. 1998. Listeria monocytogenes invasion of epithelial cells requires the MEK-1/ERK-2 mitogen-activated protein kinase pathway. Infect. Immun. 66:1106-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang, X., Y. Chen, and D. Gabuzda. 1999. ERK MAP kinase links cytokine signals to activation of latent HIV-1 infection by stimulating a cooperative interaction of AP-1 and NF-κB. J. Biol. Chem. 274:27981-27988. [DOI] [PubMed] [Google Scholar]
- 33.Zwilling, B. S., and T. K. Eisenstein. 1993. Macrophage-pathogen interactions. Marcel Dekker Inc., New York, N.Y.
- 34.Zybarth, G., N. Reiling, H. Schmidtmayerova, B. Sherry, and M. Bukrinsky. 1999. Activation-induced resistance of human macrophages to HIV-1 infection in vitro. J. Immunol. 162:400-406. [PubMed] [Google Scholar]