Abstract
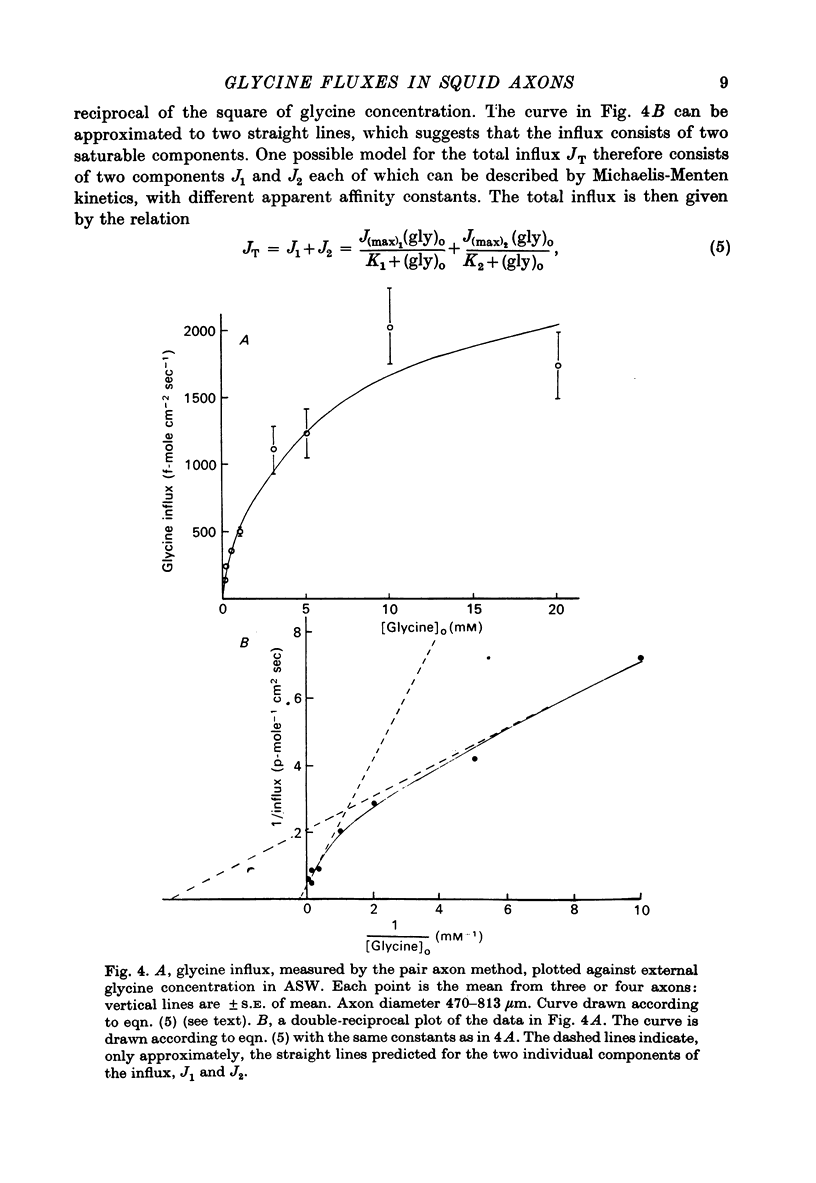
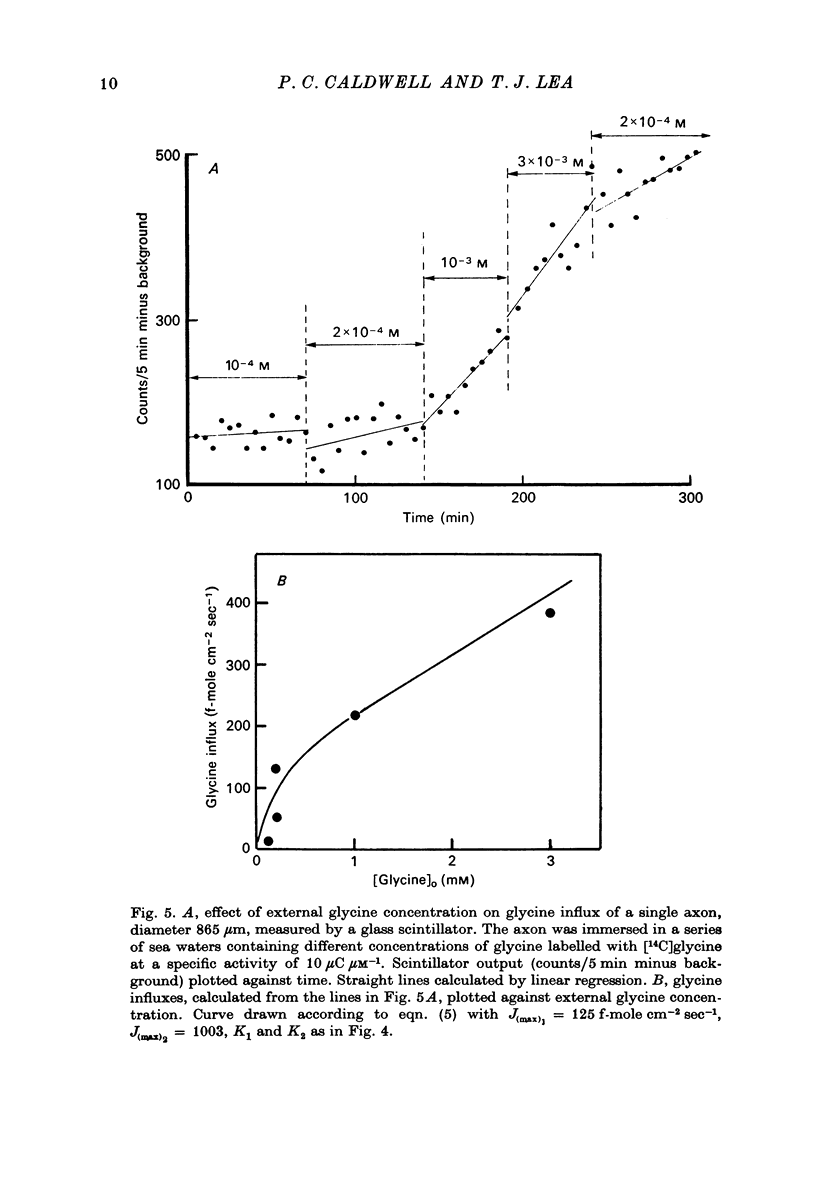
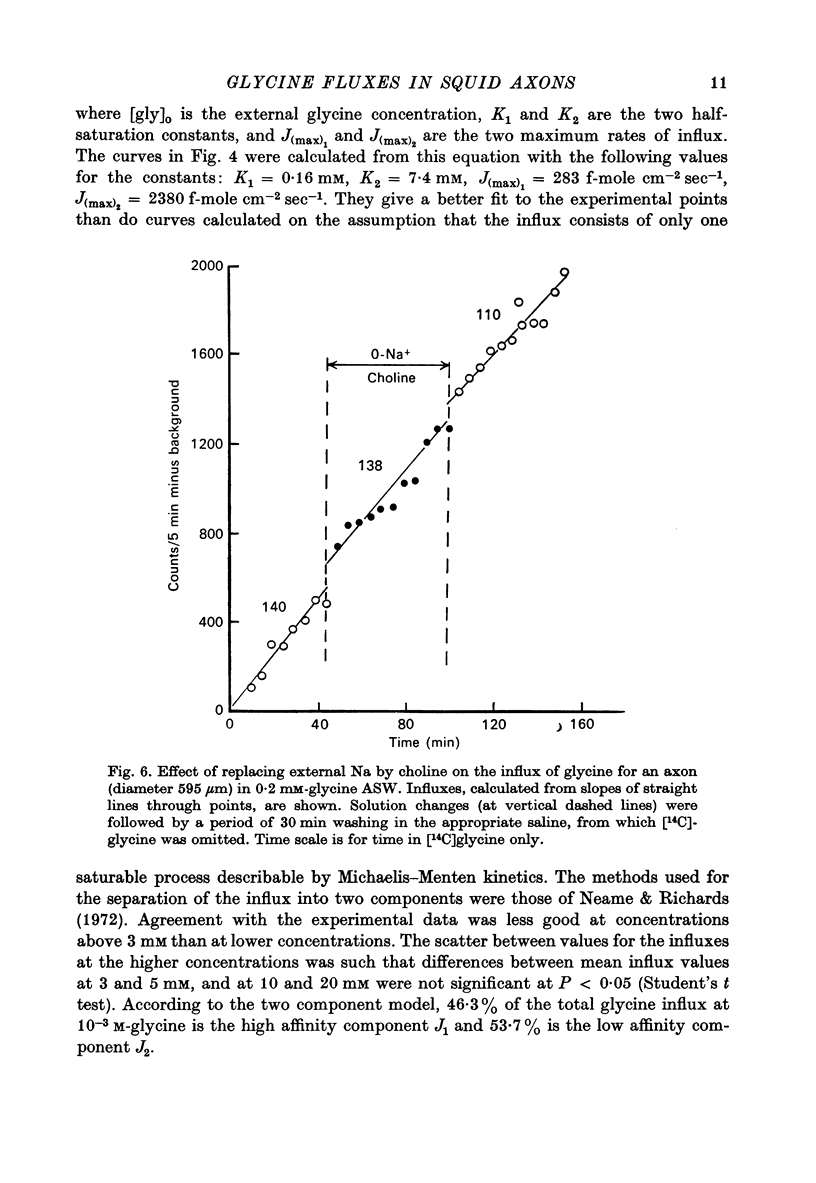
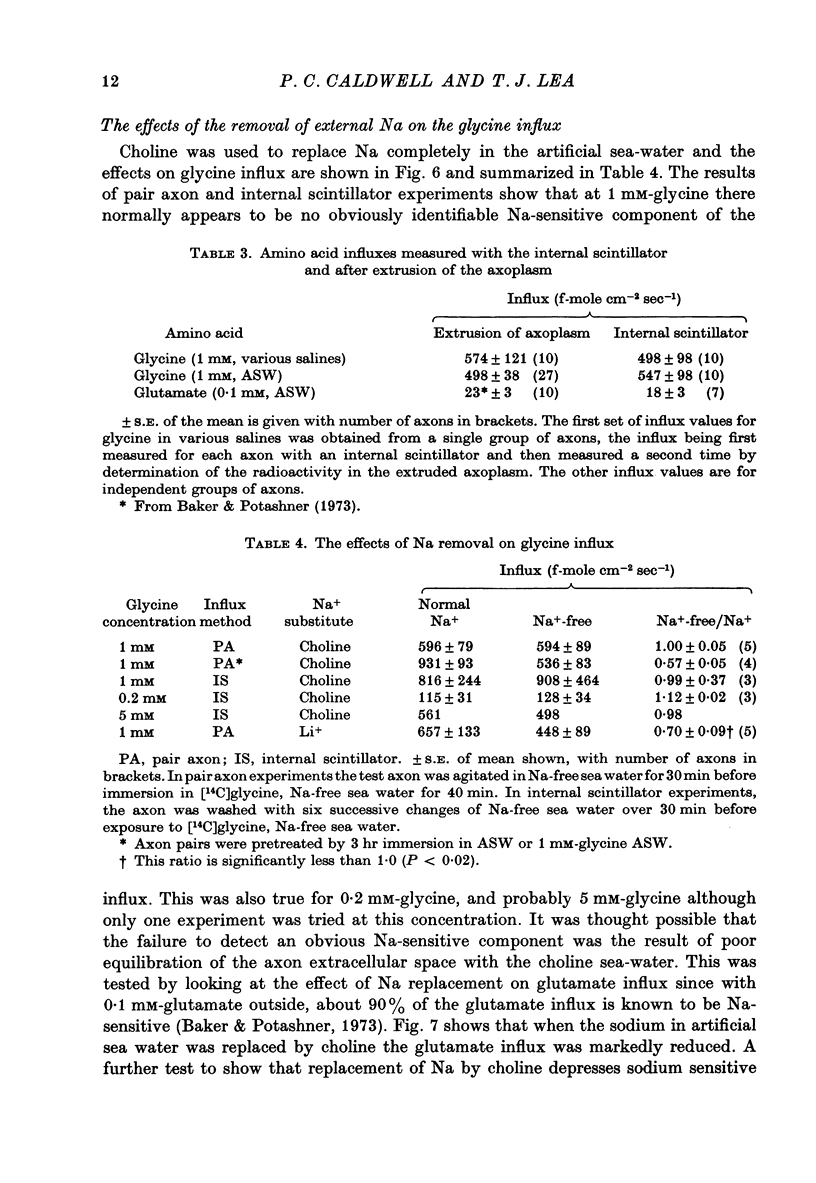
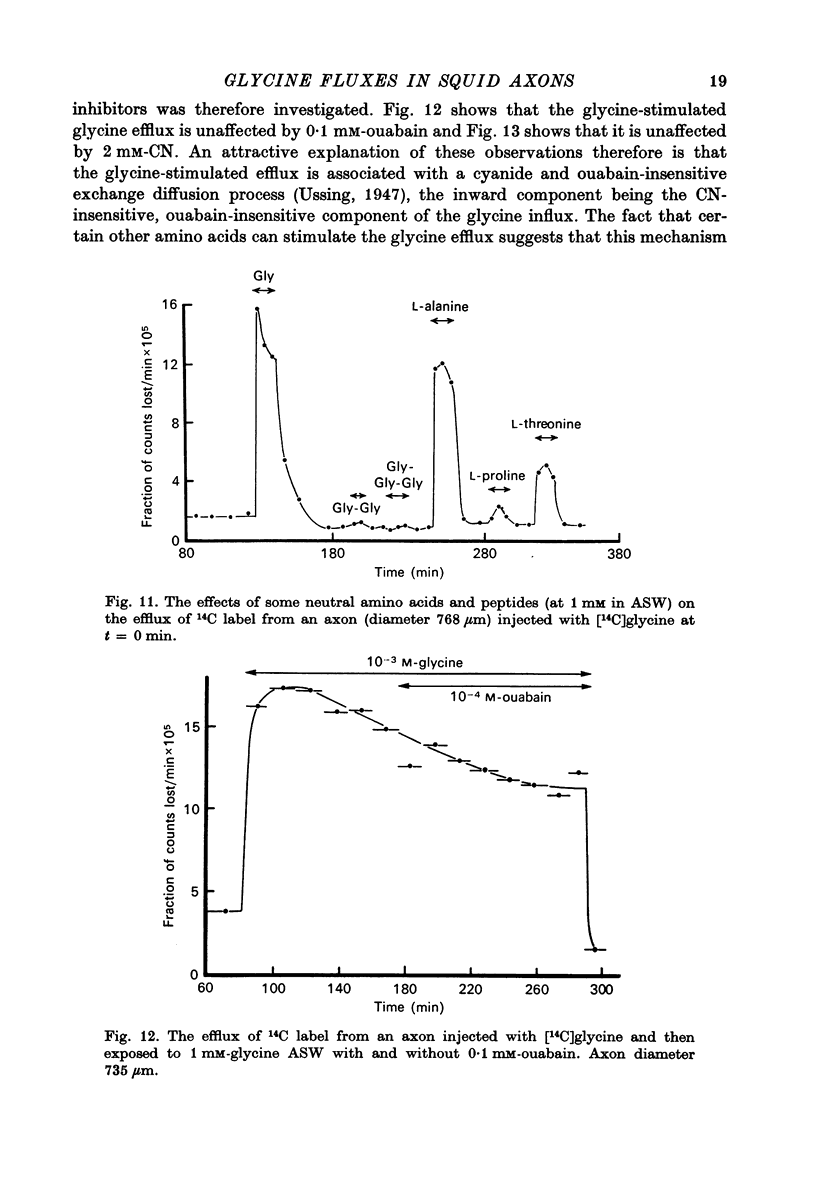
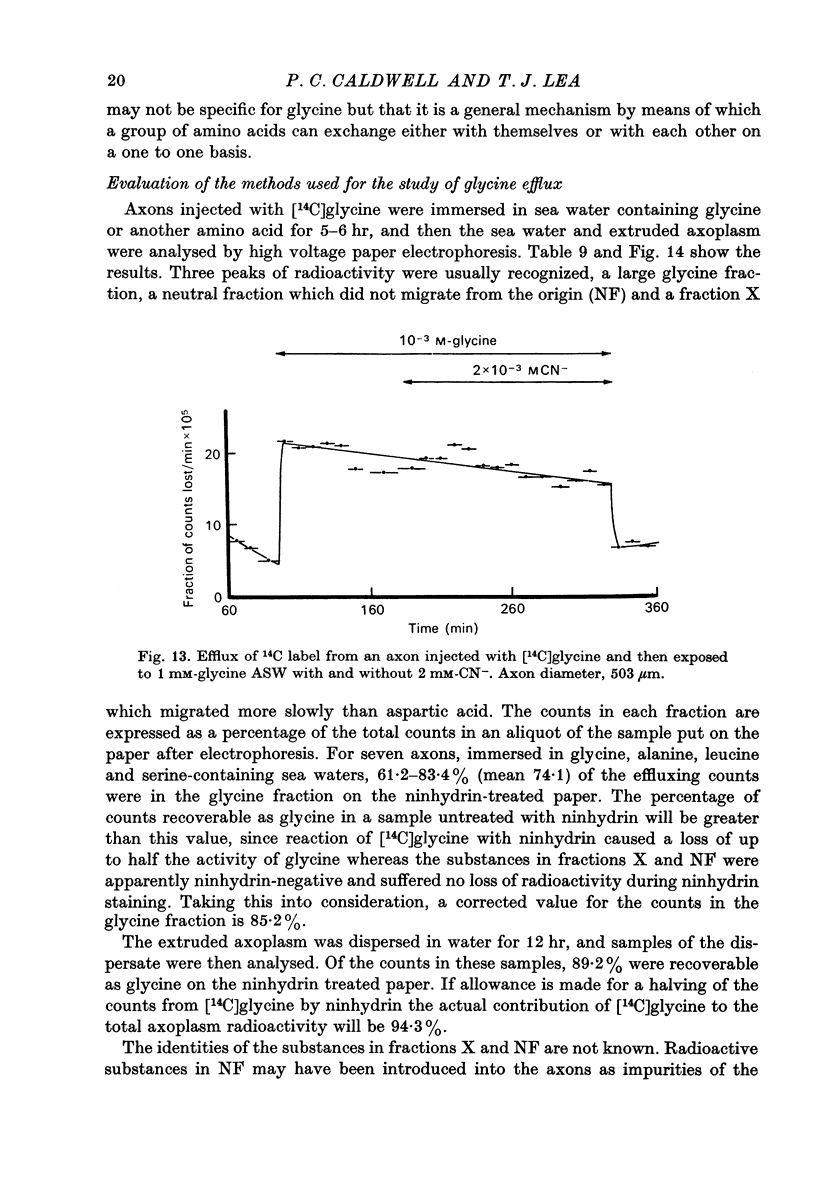
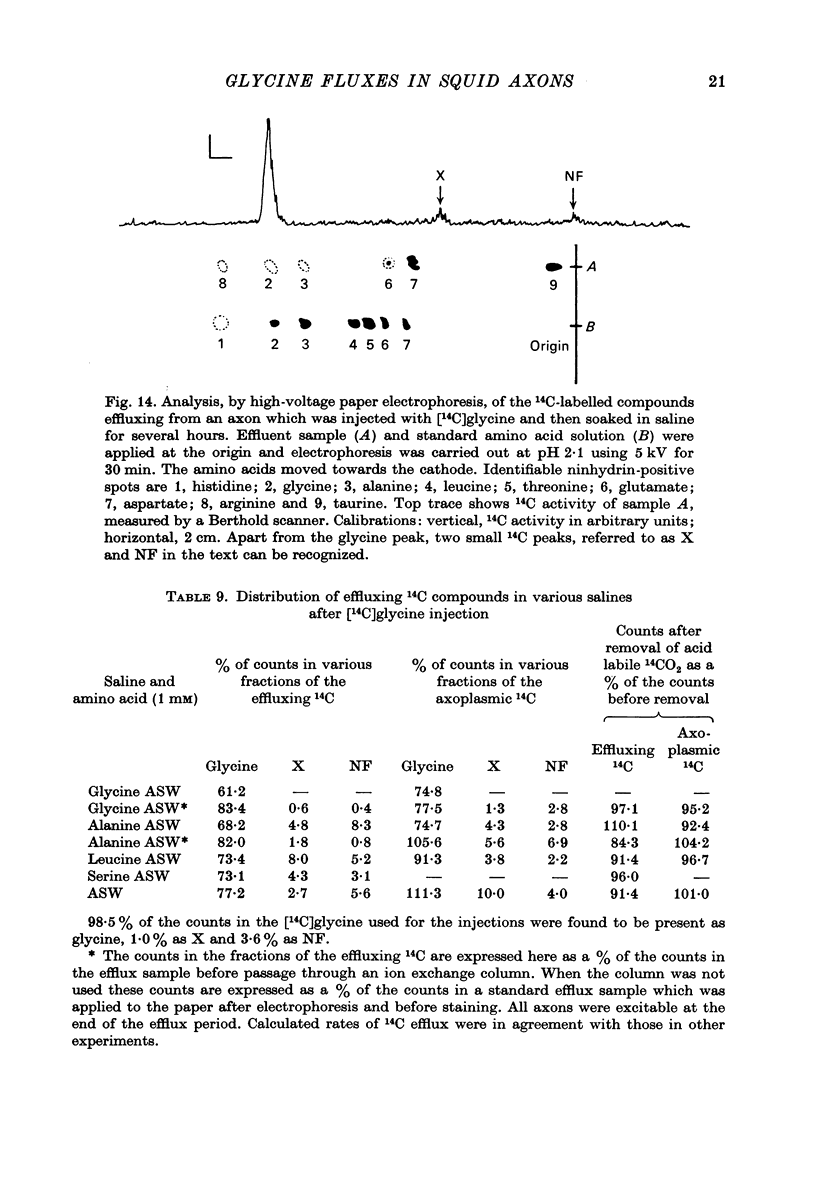
1. The influx of a number of amino acids into squid giant axons has been studied. Particular emphasis has been placed on glycine and to a lesser extent glutamate. 2. To facilitate the study of the uptake of 14C-labelled amino acids a technique was devised in which the 14C taken up was measured directly in the intact axon with a glass scintillator fibre. This technique gave results similar to the usual technique in which the axoplasm was extruded for the assay of radioactivity. 3. The changes in glycine influx with extracellular glycine concentration suggests that two saturating components are present, one with high affinity and one with low affinity. 4. The glycine influx does not seem normally to be sensitive to the removal of extracellular sodium by replacement with choline. A Na-sensitive component appeared, however, after a period of immersion in artificial sea water. There was also some depression of glycine influx if Na were replaced by Li. 5. Glutamate uptake was greatly reduced by removal of extracellular Na in confirmation of work by Baker & Potashner (1973). Orthophosphate uptake was also greatly reduced by removal of extracellular Na. 6. CN reversibly inhibited glycine uptake after a delay, indicating that part of the uptake mechanism may require ATP. 7. 14C-labelled glycine injected into squid axons was found not to exchange to any serious extent with other compounds over periods of a few hours. The glycine efflux could therefore be studied. This was found to be markedly increased by extracellular glycine and by certain other neutral amino acids applied extracellularly in the artificial sea water. 8. The enhanced glycine efflux in extracellular glycine was not affected by ouabain and CN. 9. It is suggested that glycine uptake in squid axons involves two components. One is sensitive to CN and ouabain and probably derives energy from ATP break-down. The other is probably an ATP independent exchange diffusion system in which other amino acids as well as glycine can exchange for glycine. Both these systems are independent of extracellular Na concentration. A third Na-dependent system may appear under certain conditions.
Full text
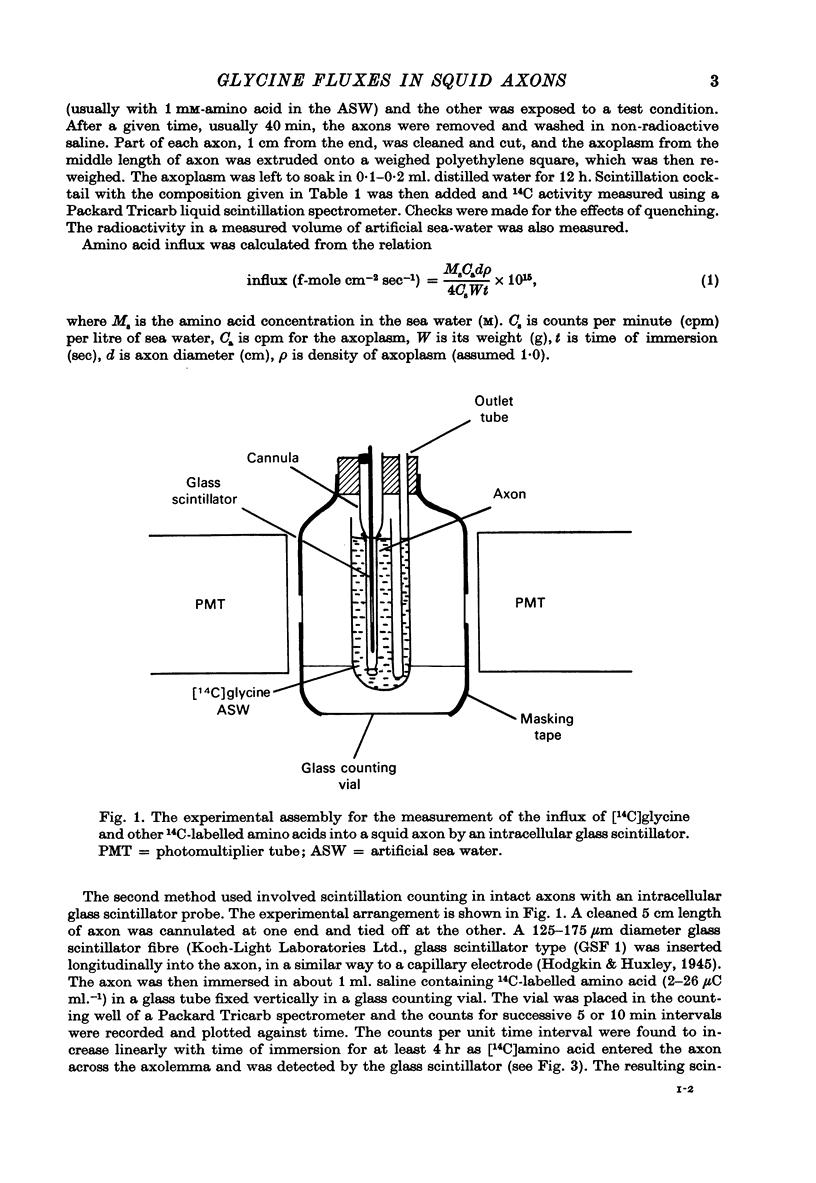
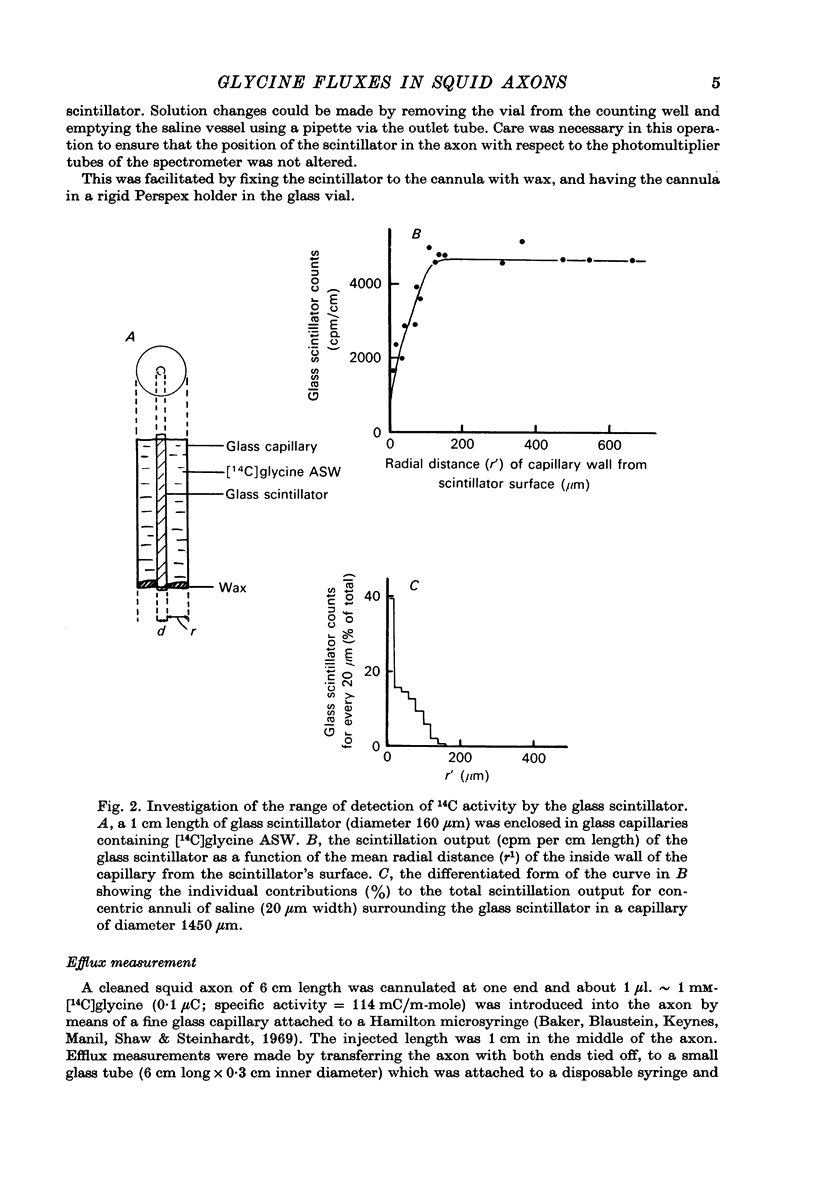
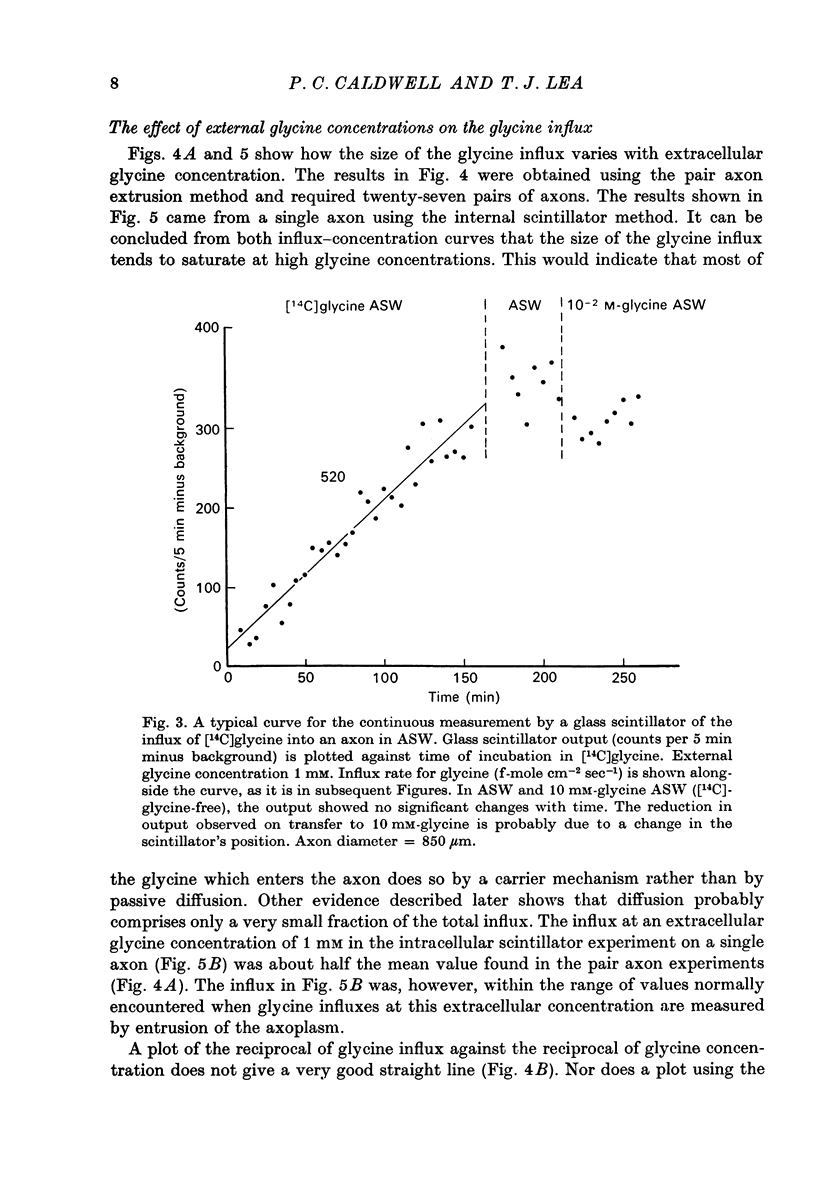
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anner B., Ferrero J., Jirounek P., Straub R. W. Uptake of orthophosphate by rabbit vagus nerve fibres. J Physiol. 1975 Jun;247(3):759–771. doi: 10.1113/jphysiol.1975.sp010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Keynes R. D., Manil J., Shaw T. I., Steinhardt R. A. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969 Feb;200(2):459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Potashner S. J. The role of metabolic energy in the transport of glutamate by invertebrate nerve. Biochim Biophys Acta. 1973 Aug 9;318(1):123–139. doi: 10.1016/0005-2736(73)90342-8. [DOI] [PubMed] [Google Scholar]
- CALDWELL P. C. The phosphorus metabolism of squid axons and its relationship to the active transport of sodium. J Physiol. 1960 Jul;152:545–560. doi: 10.1113/jphysiol.1960.sp006508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell P. C., Lea T. J. Some effects of ouabain on the transport of amino acids into squid giant axons. J Physiol. 1975 Feb;245(2):91P–92P. [PubMed] [Google Scholar]
- Caldwell P. C., Lea T. J. Use of an intercellular glass scintillator for the continuous measurement of the uptake of 14C-labelled glycine into squid giant axons. J Physiol. 1973 Jul;232(1):4P–5P. [PubMed] [Google Scholar]
- Caldwell P. C., Lowe A. G. The influx of orthophosphate into squid giant axons. J Physiol. 1970 Apr;207(2):271–280. doi: 10.1113/jphysiol.1970.sp009060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEFFNER G. G. The dialyzable free organic constituents of squid blood; a comparison with nerve axoplasm. Biochim Biophys Acta. 1961 Feb 18;47:378–388. doi: 10.1016/0006-3002(61)90298-0. [DOI] [PubMed] [Google Scholar]
- Fischer S., Litvak S. The incorporation of microinjected 14C-amino acids into TCA insoluble fractions of the giant axon of the squid. J Cell Physiol. 1967 Aug;70(1):69–74. doi: 10.1002/jcp.1040700110. [DOI] [PubMed] [Google Scholar]
- HEINZ E. Kinetic studies on the influx of glycine-1-C14 into the Ehrlich mouse ascites carcinoma cell. J Biol Chem. 1954 Dec;211(2):781–790. [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Huxley A. F. Resting and action potentials in single nerve fibres. J Physiol. 1945 Oct 15;104(2):176–195. doi: 10.1113/jphysiol.1945.sp004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskin F. C., Brande M. An improved sulphur assay applied to a problem of isethionate metabolism in squid axon and other nerves. J Neurochem. 1973 May;20(5):1317–1327. doi: 10.1111/j.1471-4159.1973.tb00243.x. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The sodium and potassium content of cephalopod nerve fibers. J Physiol. 1951 Jun;114(1-2):151–182. doi: 10.1113/jphysiol.1951.sp004609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OXENDER D. L., CHRISTENSEN H. N. DISTINCT MEDIATING SYSTEMS FOR THE TRANSPORT OF NEUTRAL AMINO ACIDS BY THE EHRLICH CELL. J Biol Chem. 1963 Nov;238:3686–3699. [PubMed] [Google Scholar]
- Rosenberg P., Khairallah E. A. Effect of phospholipases A and C on free amino acid content of the squid axon. J Neurochem. 1974 Jul;23(1):55–64. doi: 10.1111/j.1471-4159.1974.tb06915.x. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]


