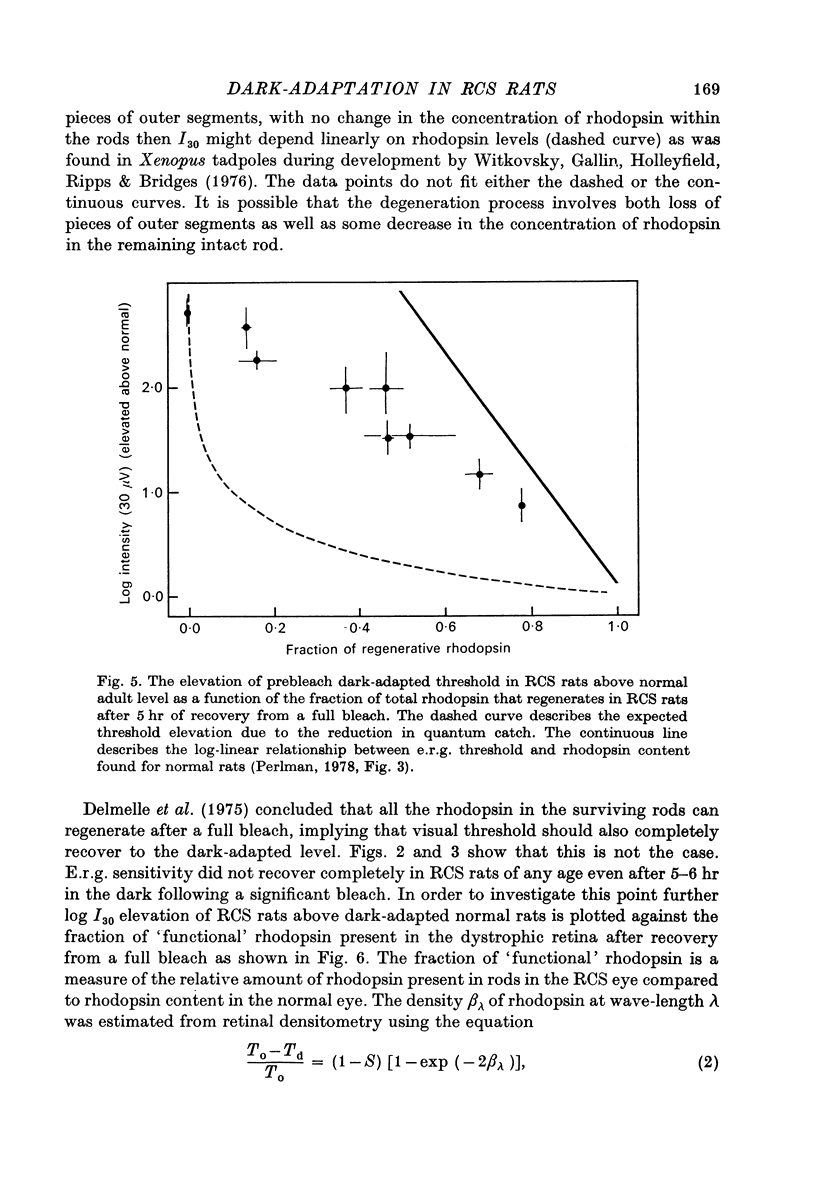
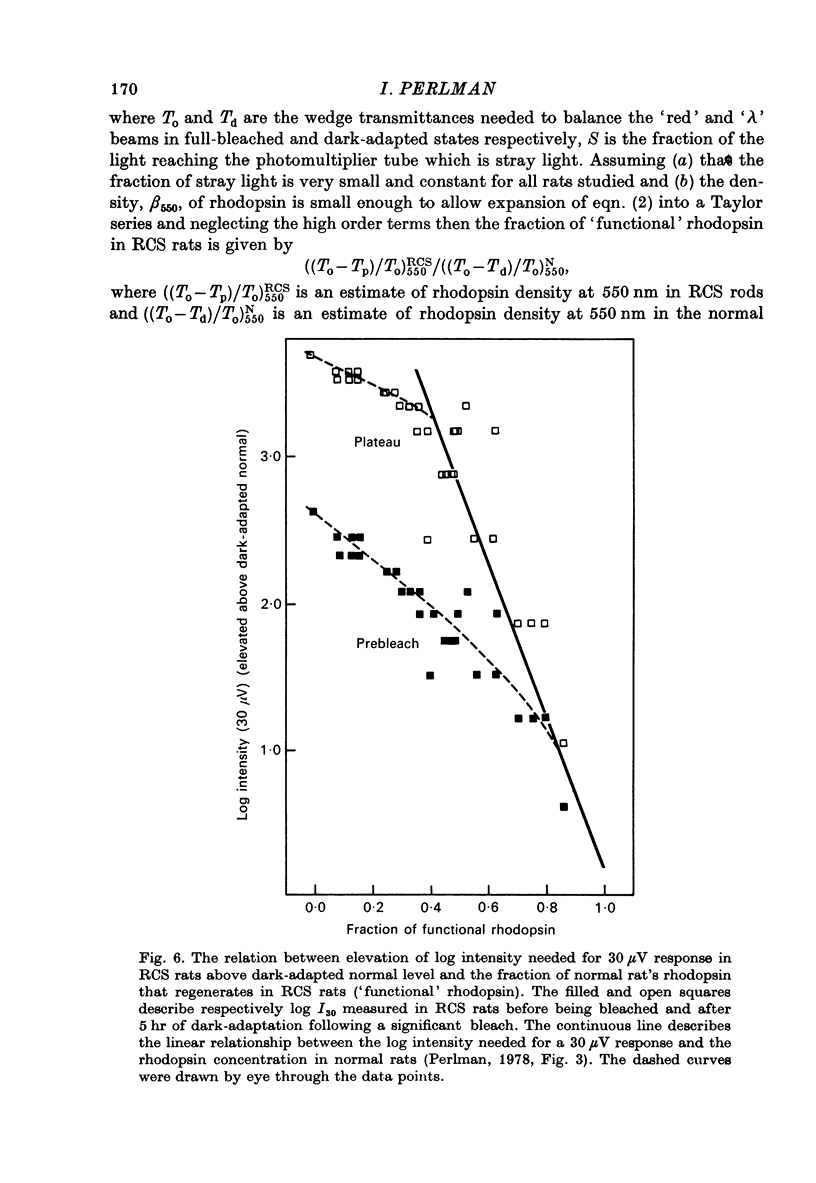
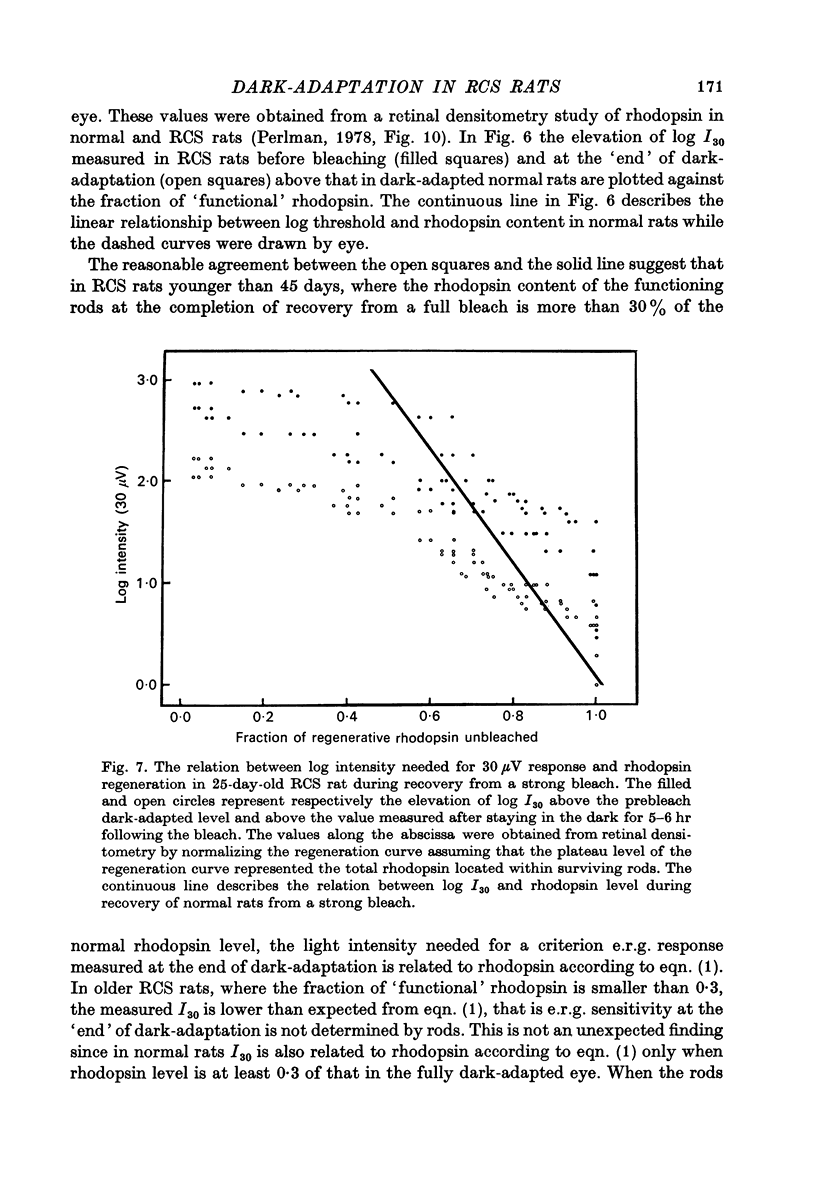
Abstract
1. Electroretinogram (e.r.g.) responses recorded from dark-reared rats with inherited retinal dystrophy (RCS) showed progressive decline in b-wave ampliture and prolongation of the time to the peak of the b-wave with age when compared with records obtained from dark-reared normal albino rats. 2. Dark-adaptation was followed in RCS and normal rats by recording the light intensity needed to evoke a criterion e.r.g. response at different time intervals after bleaching and 90% of the rhodopsin. 3. In normal rats, dark-adaptation was governed by two mechanisms. The first 25--35 min of recovery was determined by cones. The second branch, determined by the recovery of rods, lasted for about 3 hr and proceeded along an exponential time course with time constant of 41.4 +/- 2.4 min (S.E. of mean). 4. In RCS rats, the time course of the dark-adaptation after a 90% bleach depended on age. In 25--30 day old rats the recovery curve had at least three breaks separating three different mechanisms. Rats, 35--40 days old, exhibited double exponential recovery curves, while 45--70 day old rats recovered along a single exponential curve similar in time course to the cone branch of dark-adaptation found in normal rats. 5. Action spectra obtained from RCS rats at different time intervals of the recovery curve showed that in young rats, 25--30 days old, small e.r.g. responses recorded before bleaching and at the end of the recovery period were determined by rhodopsin while those recorded during the first part of the recovery from 90% bleach were determined by a combination of rods and cones. In RCS rats of advanced age (45--70 days old), rhodopsin was the major contributor to the e.r.g. responses recorded either before bleaching or at the end of the recovery period. 6. The gradual deterioration with age of the e.r.g. in RCS rats cannot be explained by either the decrease in quantum catch due to the decrease in rhodopsin content or by the linear relationship between log e.r.g. threshold and pigment concentration. 7. Using estimates of rhodopsin density within surviving rods obtained from retinal densitometry, it was shown that in RCS rats where more than 30% of normal levels of rhodopsin was located within the functioning rods, the log intensity needed for a criterion e.r.g. response measured at the end of the recovery period from a 90% bleach was linearly related to the fraction of 'functional' rhodopsin. 8. No simple relationship between log e.r.g. threshold and rhodopsin concentration could be found during the course of recovery in the dark from a strong bleaching exposure in RCS rats of all ages.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern M. Rhodopsin kinetics in the human eye. J Physiol. 1971 Sep;217(2):447–471. doi: 10.1113/jphysiol.1971.sp009580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson E. L., Gouras P., Gunkel R. D., Myrianthopoulos N. C. Dominant retinitis pigmentosa with reduced penetrance. Arch Ophthalmol. 1969 Feb;81(2):226–234. doi: 10.1001/archopht.1969.00990010228013. [DOI] [PubMed] [Google Scholar]
- Berson E. L., Gouras P., Gunkel R. D., Myrianthopoulos N. C. Rod and cone responses in sex-linked retinitis pigmentosa. Arch Ophthalmol. 1969 Feb;81(2):215–225. doi: 10.1001/archopht.1969.00990010217012. [DOI] [PubMed] [Google Scholar]
- Berson E. L., Gouras P., Gunkel R. D. Rod responses in retinitis pigmentosa, dominantly inherited. Arch Ophthalmol. 1968 Jul;80(1):58–67. doi: 10.1001/archopht.1968.00980050060009. [DOI] [PubMed] [Google Scholar]
- Berson E. L., Gouras P., Hoff M. Temporal aspects of the electroretinogram. Arch Ophthalmol. 1969 Feb;81(2):207–214. doi: 10.1001/archopht.1969.00990010209011. [DOI] [PubMed] [Google Scholar]
- Berson E. L., Kanters L. Cone and rod responses in a family with recessively inherited retinitis pigmentosa. Arch Ophthalmol. 1970 Sep;84(3):288–297. doi: 10.1001/archopht.1970.00990040290006. [DOI] [PubMed] [Google Scholar]
- Birch D., Jacobs G. H. Behavioral measurements of rat spectral sensitivity. Vision Res. 1975 Jun;15(6):687–691. doi: 10.1016/0042-6989(75)90285-0. [DOI] [PubMed] [Google Scholar]
- CONE R. A. QUANTUM RELATIONS OF THE RAT ELECTRORETINOGRAM. J Gen Physiol. 1963 Jul;46:1267–1286. doi: 10.1085/jgp.46.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicerone C. M. Cones survive rods in the light-damaged eye of the albino rat. Science. 1976 Dec 10;194(4270):1183–1185. doi: 10.1126/science.996550. [DOI] [PubMed] [Google Scholar]
- DOWLING J. E. Chemistry of visual adaptation in the rat. Nature. 1960 Oct 8;188:114–118. doi: 10.1038/188114a0. [DOI] [PubMed] [Google Scholar]
- DOWLING J. E., SIDMAN R. L. Inherited retinal dystrophy in the rat. J Cell Biol. 1962 Jul;14:73–109. doi: 10.1083/jcb.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmelle M., Noell W. K., Organisciak D. T. Hereditary retinal dystrophy in the rat: rhodopsin, retinol, vitamin A deficiency. Exp Eye Res. 1975 Oct;21(4):369–380. doi: 10.1016/0014-4835(75)90047-0. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. Visual adaptation in the retina of the skate. J Gen Physiol. 1970 Oct;56(4):491–520. doi: 10.1085/jgp.56.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. G. Light adaptation in the rat retina: evidence for two receptor mechanisms. Science. 1971 Nov 5;174(4009):598–600. doi: 10.1126/science.174.4009.598. [DOI] [PubMed] [Google Scholar]
- Green D. G. Scotopic and photopic components of the rat electroetinogram. J Physiol. 1973 Feb;228(3):781–797. doi: 10.1113/jphysiol.1973.sp010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollins M., Alpern M. Dark adaptation and visual pigment regeneration in human cones. J Gen Physiol. 1973 Oct;62(4):430–447. doi: 10.1085/jgp.62.4.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T., Funahashi M. Light effect on the synaptic organ of the rat. Invest Ophthalmol. 1976 May;15(5):407–411. [PubMed] [Google Scholar]
- Kuwabara T., Gorn R. A. Retinal damage by visible light. An electron microscopic study. Arch Ophthalmol. 1968 Jan;79(1):69–78. doi: 10.1001/archopht.1968.03850040071019. [DOI] [PubMed] [Google Scholar]
- Perlman I. Kinetics of bleaching and regeneration of rhodopsin in abnormal (RCS) and normal albino rats in vivo. J Physiol. 1978 May;278:141–159. doi: 10.1113/jphysiol.1978.sp012297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh E. N. Rhodopsin flash photolysis in man. J Physiol. 1975 Jun;248(2):393–412. doi: 10.1113/jphysiol.1975.sp010981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. Rhodopsin measurement and dark-adaptation in a subject deficient in cone vision. J Physiol. 1961 Apr;156:193–205. doi: 10.1113/jphysiol.1961.sp006668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P., Gallin E., Hollyfield J. G., Ripps H., Bridges C. D. Photoreceptor thresholds and visual pigment levels in normal and vitamin A-deprived Xenopus tadpoles. J Neurophysiol. 1976 Nov;39(6):1272–1287. doi: 10.1152/jn.1976.39.6.1272. [DOI] [PubMed] [Google Scholar]


