Abstract
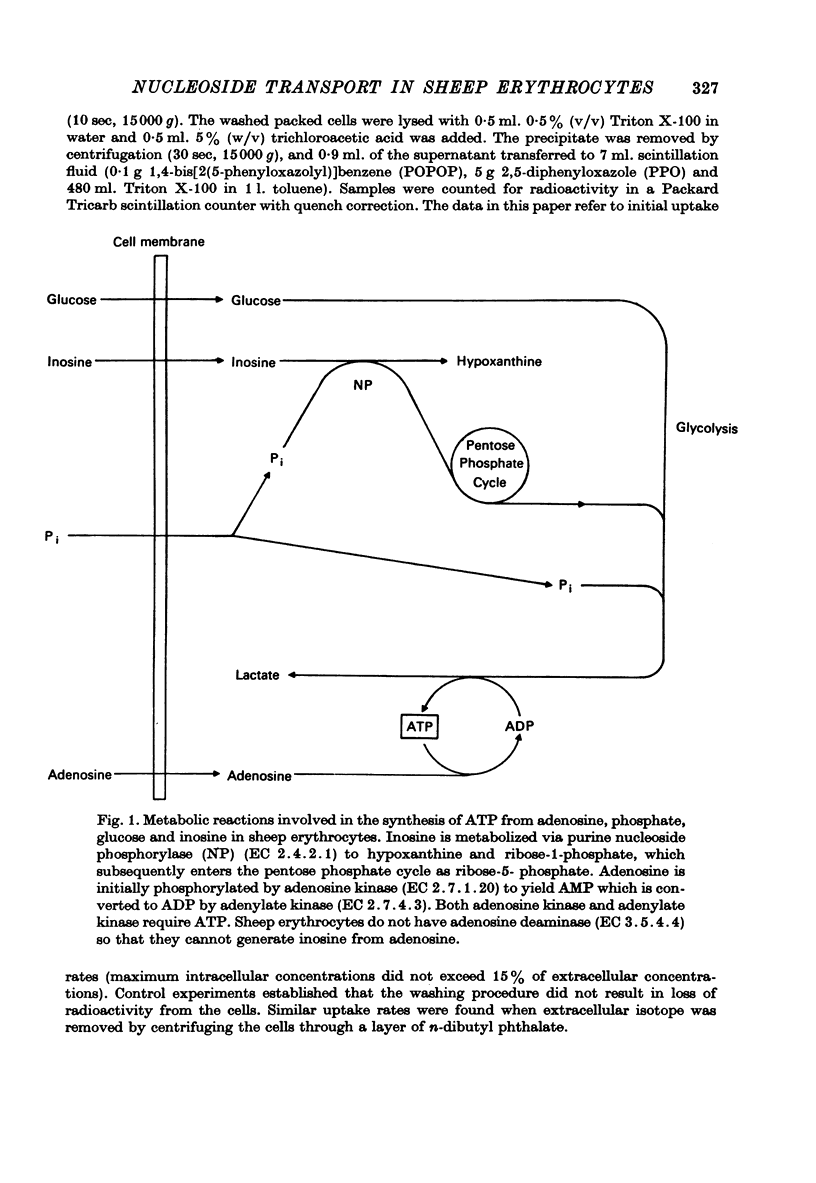
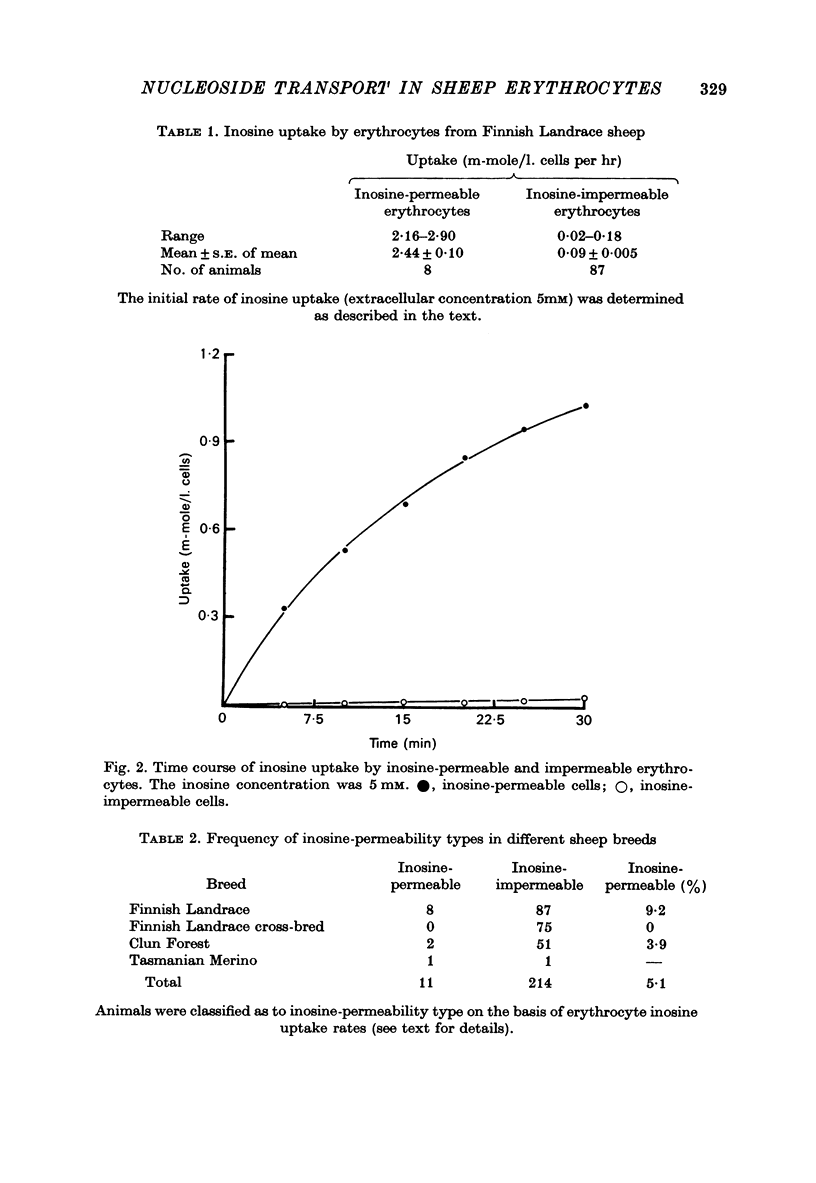
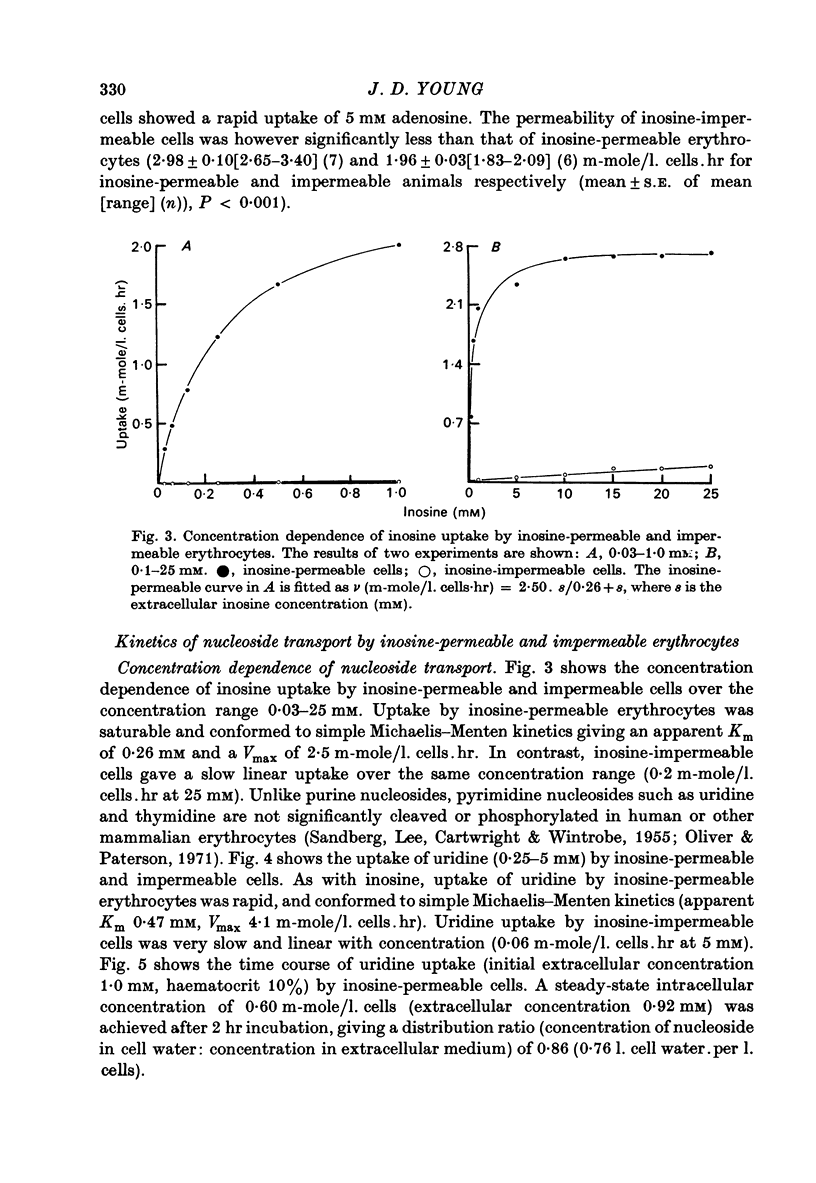
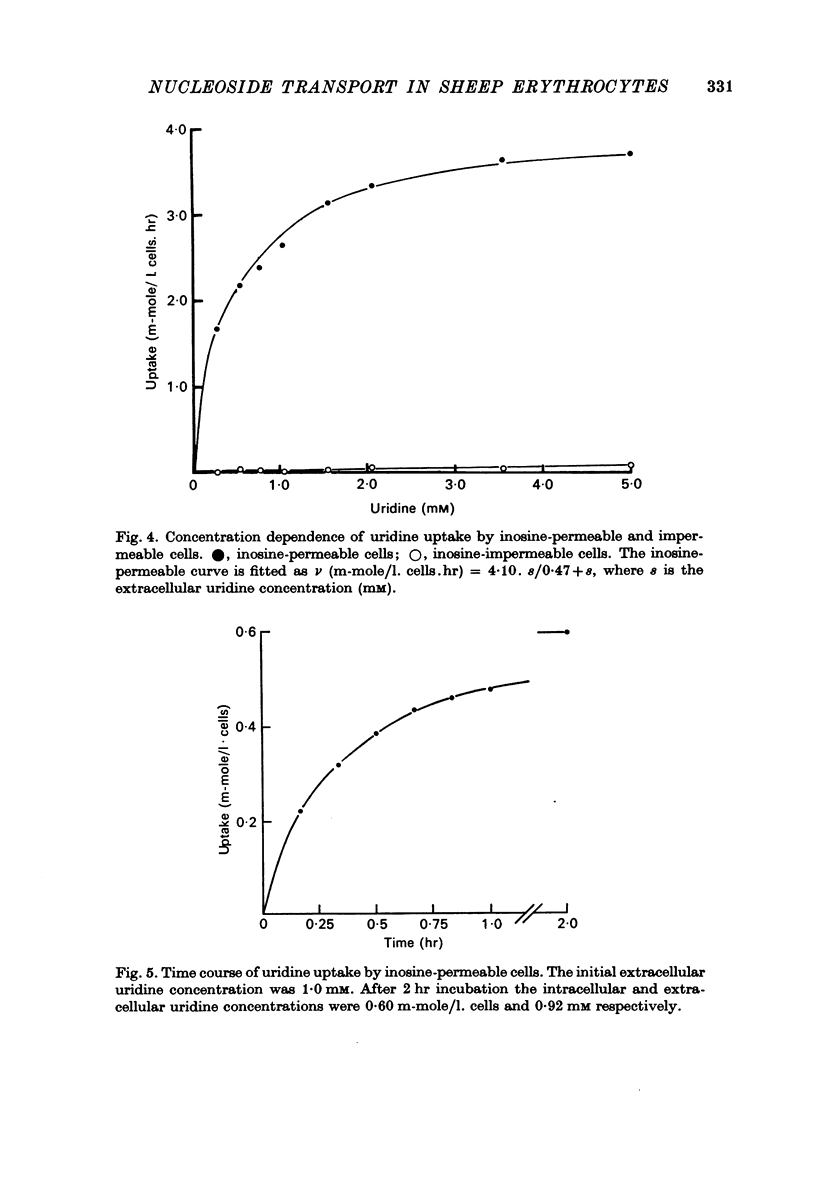
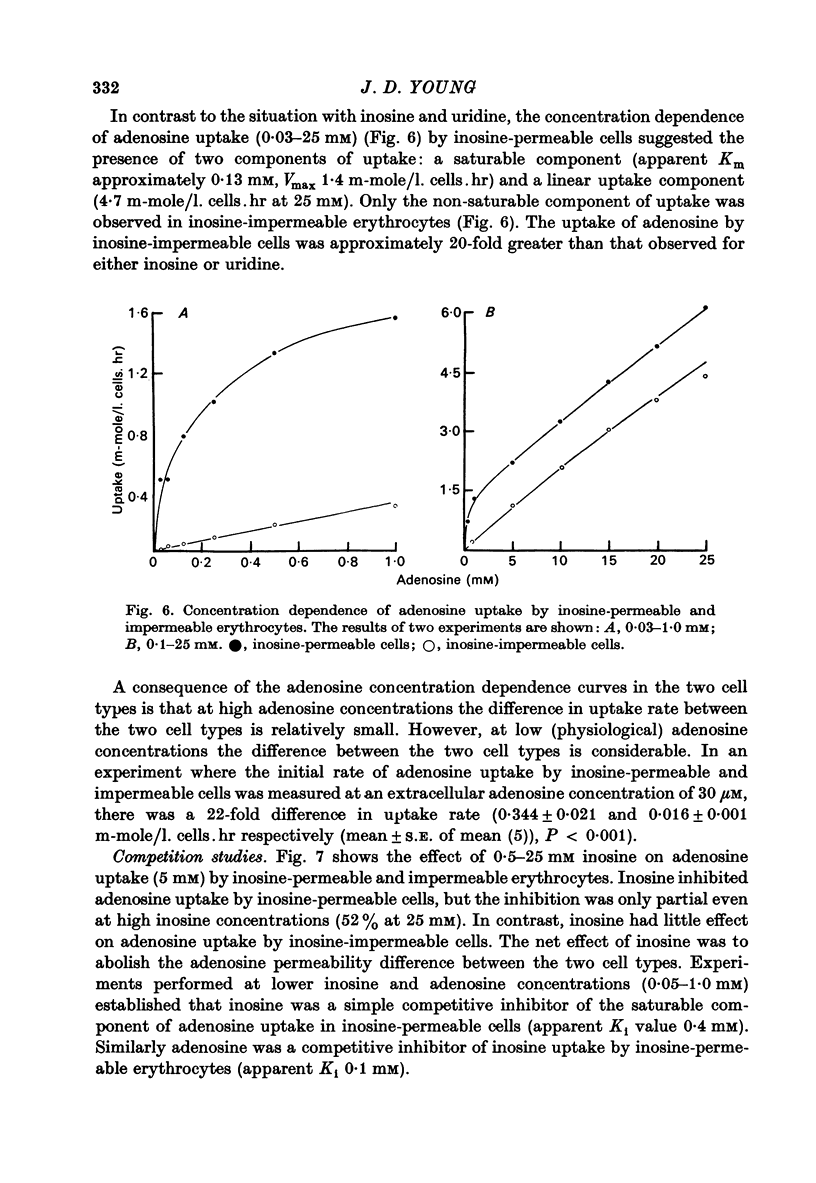
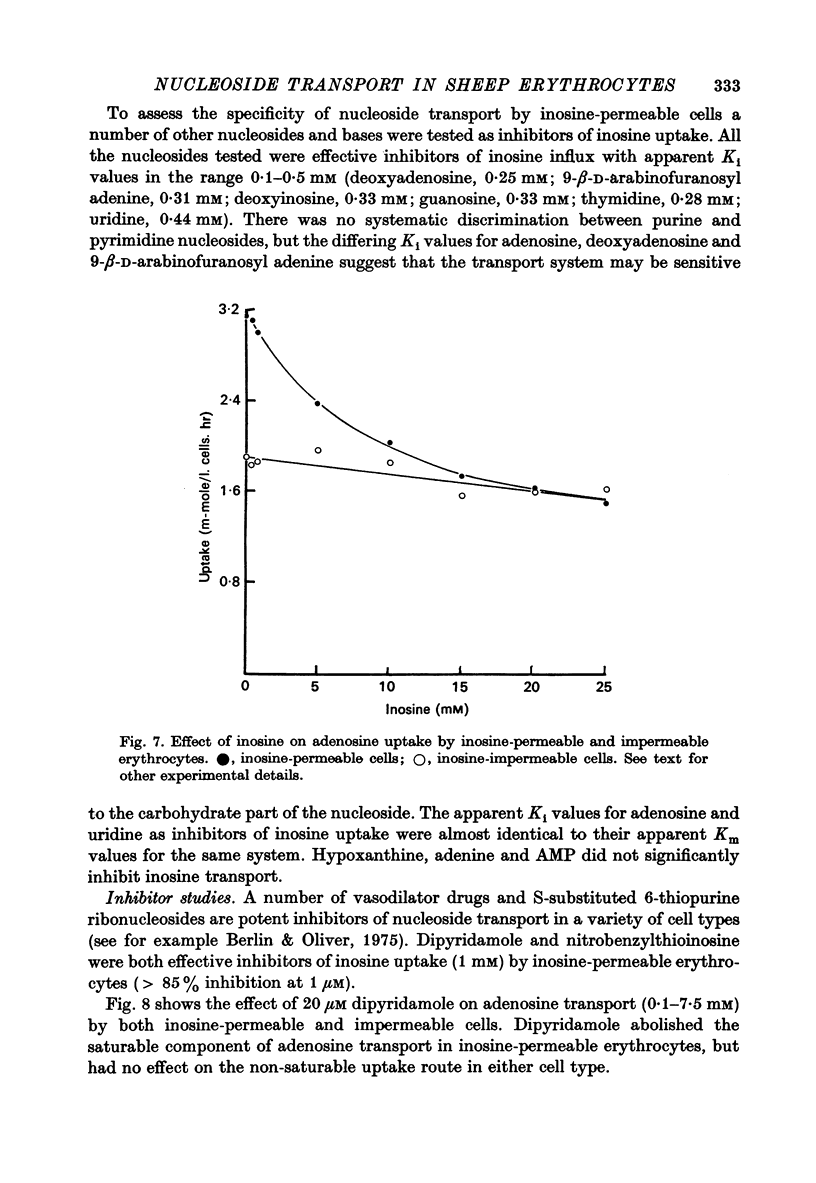
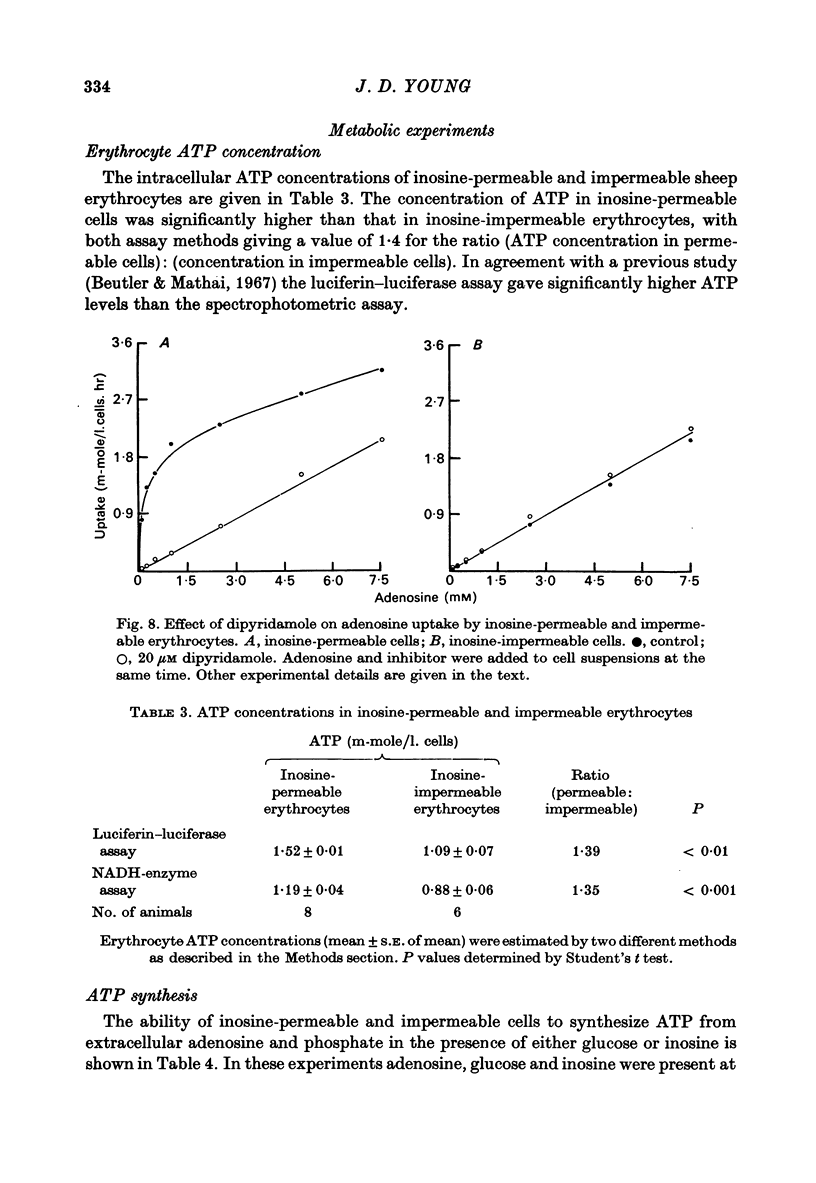
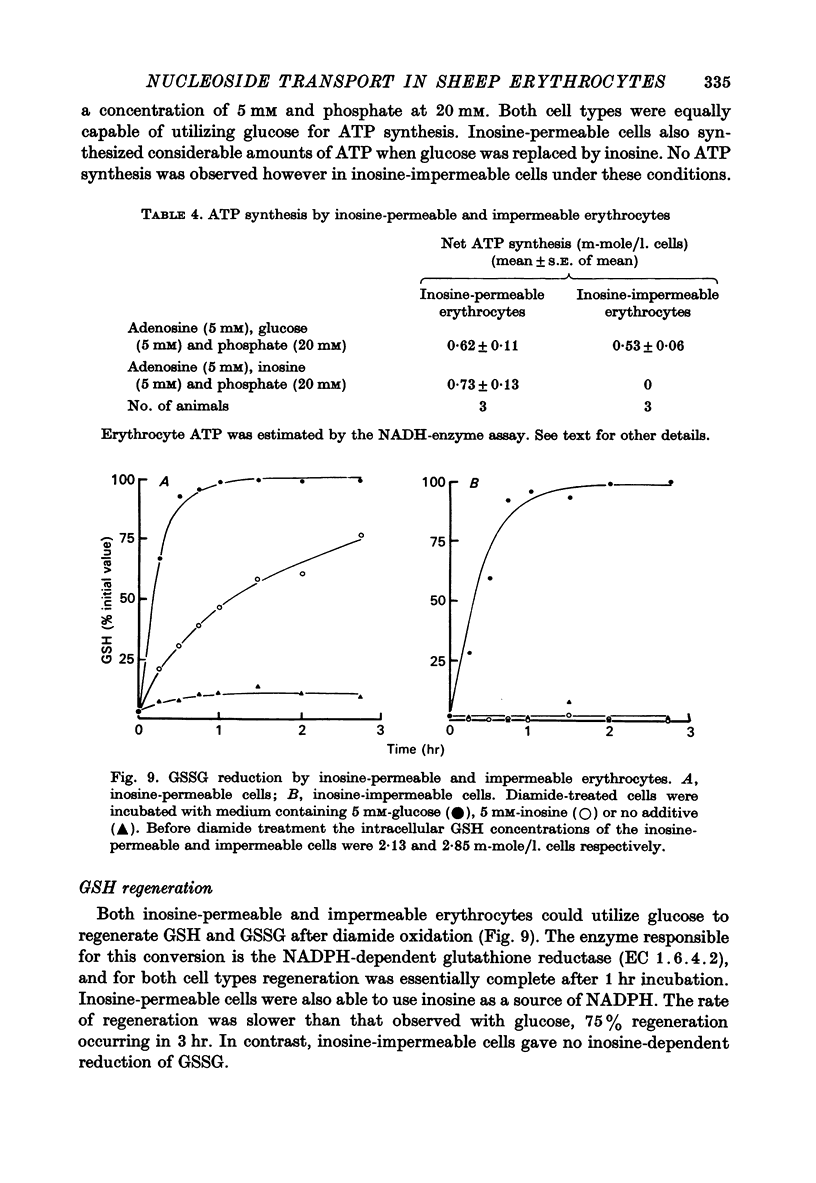
1. The permeability of sheep erythrocytes to purine and pyrimidine nucleosides was investigated. Erythrocytes from most sheep (nucleoside-impermeable) were almost completely impermeable to 5 mM inosine whereas cells from approximately 5% of the animals studied (nucleoside-permeable) showed a rapid inosine uptake. Cells from both types of animal were permeable to 5 mM adenosine, although transport was slower in nucleoside-impermeable erythrocytes. 2. Two distinct nucleoside transport routes were present in nucleoside-permeable erythrocytes; a high affinity (apparent Km congruent to 0.2 mM) facilitated diffusion system which transported both purine and pyrimidine nucleosides, and a non-saturable uptake route selective for adenosine. The high affinity system was the major route of adenosine transport at physiological concentrations. 3. Transport by the high affinity system was completely inhibited by micromolar concentrations of dipyridamole and nitrobenzylthioinosine. Dipyridamole had no effect on the non-saturable component of adenosine uptake. 4. The transport differences between nucleoside-permeable and impermeable erythrocytes were due to the absence of the high affinity system from nucleoside-impermeable cells. 5. Nucleoside-permeable cells had a higher intracellular ATP concentration than nucleoside-impermeable erythrocytes, suggesting that the high affinity transport system participates in the energy metabolism of the cell.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEUTLER E., DURON O., KELLY B. M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882–888. [PubMed] [Google Scholar]
- Brinkley B. R., Hittelman W. N. Ultrastructure of mammalian chromosome aberrations. Int Rev Cytol. 1975;42:49–101. doi: 10.1016/s0074-7696(08)60978-x. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Ginsburg H. Transport of uridine in human red blood cells. Demonstration of a simple carrier-mediated process. J Gen Physiol. 1977 Jan;69(1):75–96. doi: 10.1085/jgp.69.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass C. E., Gaudette L. A., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Specific binding of the inhibitor nitrobenzylthioinosine to nucleoside transport sites in the erythrocyte membrane. Biochim Biophys Acta. 1974 Apr 12;345(1):1–10. doi: 10.1016/0005-2736(74)90239-9. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Mediated transport of nucleosides by human erythrocytes. Specificity toward purine nucleosides as permeants. Biochim Biophys Acta. 1973 Feb 16;291(3):734–746. doi: 10.1016/0005-2736(73)90477-x. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Accelerative exchange diffusion of uridine and thymidine and specificity toward pyrimidine nucleosides as permeants. J Biol Chem. 1972 May 25;247(10):3314–3320. [PubMed] [Google Scholar]
- Kim H. D., McManus T. J. Studies on the energy metabolism of pig red cells. I. The limiting role of membrane permeability in glycolysis. Biochim Biophys Acta. 1971 Jan 26;230(1):1–11. doi: 10.1016/0304-4165(71)90048-1. [DOI] [PubMed] [Google Scholar]
- Kim H. D., McManus T. J. Studies on the energy metabolism of pig red cells. II. Lactate formation from free ribose and deoxyribose with maintenance of ATP. Biochim Biophys Acta. 1971 Jan 26;230(1):12–19. doi: 10.1016/0304-4165(71)90049-3. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wertheim B., Correa W. S. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969 Nov 6;37(4):593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- LOWY B. A., RAMOT B., LONDON I. M. Adenosine triphosphate metabolism in the rabbit erythrocyte in vivo. Nature. 1958 Feb 1;181(4605):324–326. doi: 10.1038/181324a0. [DOI] [PubMed] [Google Scholar]
- LOWY B. A., RAMOT B., LONDON I. M. The biosynthesis of adenosine triphosphate and guanosine triphosphate in the rabbit erythrocyte in vivo and in vitro. J Biol Chem. 1960 Oct;235:2920–2923. [PubMed] [Google Scholar]
- Lieu T. S., Hudson R. A., Brown R. K., White B. C. Transport of pyrimidine nucleosides across human erythrocyte membranes. Biochim Biophys Acta. 1971 Sep 14;241(3):885–893. [PubMed] [Google Scholar]
- Meyskens F. L., Williams H. E. Adenosine metabolism in human erythrocytes. Biochim Biophys Acta. 1971 Jun 30;240(2):170–179. doi: 10.1016/0005-2787(71)90654-x. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Paterson A. R. Nucleoside transport. I. A mediated process in human erythrocytes. Can J Biochem. 1971 Feb;49(2):262–270. doi: 10.1139/o71-038. [DOI] [PubMed] [Google Scholar]
- Pickard M. A., Brown R. R., Paul B., Paterson A. R. Binding of the nucleoside transport inhibitor 4-nitrobenzylthioinosine to erythrocyte membranes. Can J Biochem. 1973 May;51(5):666–672. doi: 10.1139/o73-083. [DOI] [PubMed] [Google Scholar]
- Pickard M. A., Paterson A. R. Fractionation of human erythrocyte membranes. Presence of the nucleoside transport complex in an insoluble residue. Biochim Biophys Acta. 1976 Dec 14;455(3):817–823. doi: 10.1016/0005-2736(76)90051-1. [DOI] [PubMed] [Google Scholar]
- Pickard M. A., Paterson A. R. Nucleoside transport in human erythrocytes: equilibrium exchange diffusion of uridine. Can J Biochem. 1972 Jun;50(6):704–705. doi: 10.1139/o72-096. [DOI] [PubMed] [Google Scholar]
- Pickard M. A., Paterson A. R. Use of 4-nitrobenzylthioinosine in the measurement of rates of nucleoside transport in human erythrocytes. Can J Biochem. 1972 Jul;50(7):839–840. doi: 10.1139/o72-116. [DOI] [PubMed] [Google Scholar]
- Roos H., Pfleger K. Kinetics of adenosine uptake by erythrocytes, and the influence of dipyridamole. Mol Pharmacol. 1972 Jul;8(4):417–425. [PubMed] [Google Scholar]
- SANDBERG A. A., LEE G. R., CARTWRIGHT G. E., WINTROBE M. M. Purine nucleoside phosphorylase activity of blood. I. Erythrocytes. J Clin Invest. 1955 Dec;34(12):1823–1829. doi: 10.1172/JCI103238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker E. M., Young J. D. Genetic variation in the purine nucleoside phosphorylase activity of sheep red cells. Anim Blood Groups Biochem Genet. 1976;7(2):109–117. doi: 10.1111/j.1365-2052.1976.tb01384.x. [DOI] [PubMed] [Google Scholar]
- WHITTAM R. The high permeability of human red cells to adenine and hypoxanthine and their ribosides. J Physiol. 1960 Dec;154:614–623. doi: 10.1113/jphysiol.1960.sp006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Ellory J. C. Substrate specificity of amino acid transport in sheep erythrocytes. Biochem J. 1977 Jan 15;162(1):33–38. doi: 10.1042/bj1620033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Ellory J. C., Tucker E. M. Amino acid transport in normal and glutathione-deficient sheep erythrocytes. Biochem J. 1976 Jan 15;154(1):43–48. doi: 10.1042/bj1540043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Nimmo I. A., Hall J. G. The relationship between GSH, GSSG and non-GSH thiol in GSH-deficient erythrocytes from Finnish landrace and Tasmanian merino sheep. Biochim Biophys Acta. 1975 Sep 8;404(1):124–131. doi: 10.1016/0304-4165(75)90153-1. [DOI] [PubMed] [Google Scholar]
- Young J. D. Nucleoside transport in inosine-permeable and impermeable sheep erythrocytes [proceedings]. J Physiol. 1977 Jul;269(1):46P–47P. [PubMed] [Google Scholar]
- Young J. D. Proceedings: Nucleoside transport variation in sheep erythrocytes. J Physiol. 1976 Jul;259(1):57P–58P. [PubMed] [Google Scholar]


