Abstract
Human salivary histatin 5 (Hst 5) is a nonimmune salivary protein with antifungal activity against an important human pathogen, Candida albicans. The candidacidal activity of histatins appears to be a distinctive multistep mechanism involving depletion of the C. albicans intracellular ATP content as a result of nonlytic ATP efflux. Hst 5 caused a loss of cell viability concomitant with a decrease in cellular volume as determined both by a classical candidacidal assay with exogenous Hst 5 and by using a genetically engineered C. albicans strain expressing Hst 5. Preincubation of C. albicans cells with pharmacological inhibitors of anion transport provided complete or substantial protection from Hst 5-induced killing and volume reduction of cells. Moreover, intracellular expression of Hst 5 resulted in a reduction in the population mean cell volume that was accompanied by an increase in the percentage of unbudded cells and C. albicans cells in the G1 phase. Following expression of Hst 5, the smallest cells sorted by fluorescence-activated cell sorting from the total population did not replicate and were exclusively in the G1 phase. Cells with intracellularly expressed Hst 5 had greatly reduced G1 cyclin transcript levels, indicating that they arrested in the G1 phase before the onset of Start. Our data demonstrate that a key determinant in the mechanism of Hst 5 toxicity in C. albicans cells is the disruption of regulatory circuits for cell volume homeostasis that is closely coupled with loss of intracellular ATP. This novel process of fungicidal activity by a human salivary protein has highlighted potential interactions of Hst 5 with volume regulatory mechanisms and the process of yeast cell cycle control.
Human saliva contains nonimmune proteins with potent antimicrobial activities, including lactoperoxidase, lysozyme, lactoferrin, and histatins (Hsts). These proteins contribute to the innate host defense system in the oral cavity and have wide spectra of activities against bacteria and fungi. Hsts are a family of structurally related histidine-rich basic proteins of human acinar cell origin that possess in vitro candidacidal and candidastatic activities (21). Hst 5 (a proteolytic product of Hst 3) has the highest in vitro anticandidal activity of the family at physiological concentrations found in saliva (15 to 50 μM). The functional role of Hsts in vivo is believed to involve prevention of oral overgrowth of Candida albicans and nonimmune protection from oropharyngeal candidiasis (17). Understanding the mechanisms by which salivary Hsts exhibit candidacidal activity may lead to development of new antifungal drugs.
The candidacidal activity of Hst 5 appears to be a multistep mechanism involving extracellular binding with a 67-kDa yeast protein (7), internalization (1, 30), and possibly a final interaction with intracellular targets (12, 15). Our previous studies found that depletion of C. albicans intracellular ATP content as a result of nonlytic ATP efflux correlated with the candidacidal activity of Hst 5, as pharmacological agents or growth conditions that inhibited Hst 5-induced cell killing also reduced ATP release (14, 15). ATP efflux from C. albicans cells occurred within 5 min following Hst 5 addition while the yeast cells remained polarized and respiration continued (14). This ATP release occurred in structurally intact cells, yet the cells could not replicate, suggesting that Hst 5 induces ultimate growth arrest.
We constructed C. albicans strains that contain chromosomally encoded human salivary Hst genes under the control of a regulated promoter in order to understand the mechanism of Hst 5 action independent of binding and translocation events (1). Intracellular expression of Hst 5 resulted in a nearly 65% reduction in yeast cell growth after 24 h and induced ATP release paralleling the loss of cell viability. Thus, intracellular Hst 5 alone can cause ATP release and prevent cell growth, showing that these events can be initiated by Hst 5 from the cytosolic compartment and that extracellular binding of Hst 5 to cells is likely a translocation event. However, anaerobically grown C. albicans cells also showed depletion of intracellular ATP by way of efflux, but they were less susceptible to Hst 5 (14), suggesting that ATP release may accompany another cellular response that leads to cell death.
In higher eukaryotic cells, ATP release is triggered by hypotonic challenge, which is an essential autocrine control mechanism for cell volume regulation (26). The prominent release of cellular ATP in response to Hst 5 treatment from C. albicans cells raised the possibility that ATP release initiated by Hst 5 may be accompanied by cell volume changes. Utilizing both a classical candidacidal assay in which C. albicans cells are suspended in a buffer and treated with Hst 5 and our genetically engineered C. albicans strain expressing Hst 5, we found that Hst 5 caused a loss of cell viability concomitant with a decrease in cellular volume in both experimental groups. In addition, we found that inhibitors of anion transporter or volume-regulating Cl− channels (19), including diisothiocyanatostilbene-2,2′-disulfonic acid (disodium salt) (DIDS), 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB), and 2-(3-[trifluoromethyl]anilino)nicotinic acid (niflumic acid), provided substantial protection from Hst 5-induced killing of C. albicans, as well as Hst 5-induced volume reduction of cells.
Yeast cell size increase is a critical determinant for progression through the cell cycle. Prompted by findings of cell volume loss following Hst 5 treatment, we examined whether cell cycle arrest could be a consequence of this process. The findings reported here suggest that Hst-5 candidacidal activity may be associated with cell cycle arrest in targeted C. albicans cells. Thus, Hst 5 may combat fungal human pathogens by volume disorder and disruption of the highly ordered and regulated process of yeast cell cycle control.
MATERIALS AND METHODS
Yeast strains and culture conditions.
All C. albicans strains were maintained on Sabouraud dextrose agar plates and recultured monthly. For the classical candidacidal assay, one colony of C. albicans DS1 was inoculated into 10 ml of sucrose-salts-biotin yeast synthetic medium and grown to the stationary phase at 25°C with rotary agitation at 200 rpm. Cell numbers were determined by phase-contrast microscopy by using a cell-counting chamber. Derivatives of C. albicans SGY-243 that contain integrated genes for codon-optimized human salivary Hst 5 (strain DB9) and the coding sequence of the secreted version of the human salivary statherin gene (strain DB13) regulated by the CaMAL2 promoter region were used (1). Growth in glucose (noninducing medium) resulted in a wild-type phenotype for both DB9 and DB13, and growth in sucrose (inducing medium) resulted in expression of Hst 5 or statherin. Expression of statherin caused no reduction in the growth of C. albicans DB13 over 24 h. However, within 2 h of inoculation into sucrose-containing medium, the viability of strain DB9 was reduced by 35.6% ± 1.4%, and it was further reduced by 62.8% ± 4.7% after 23 h of induction (1). Thus, induction of expression of Hst 5 in DB9 caused a gradual increase in the loss of viability in the cell population with time and created a mixed population of viable and nonviable cells. In the present study, single colonies of C. albicans DB9 and BD13 were inoculated into 5 ml of yeast nitrogen base (YNB) medium containing 2% glucose (noninducing medium) and incubated at 37°C for 12 to 14 h with constant shaking at 250 rpm. Cells in the late log growth phase (100 μl containing 104 cells) were transferred into 100 ml of YNB medium containing 2% glucose (noninducing medium) or 2% sucrose (inducing medium) and grown for 12 to 24 h under the same conditions for expression of Hst 5 (DB9) or salivary statherin (DB13).
Candidacidal assay.
The antifungal activity of Hst 5 was examined by a microdilution plate assay (7), with the following modifications. C. albicans cells were grown in YNB, washed twice with 10 mM sodium phosphate buffer (Na2HPO4-NaH2PO4; pH 7.4), and resuspended in sodium phosphate buffer at a concentration of 2.5 × 105 cells/ml. Pharmacological agents (DIDS, NPPB, and niflumic acid) were added to 5 × 103 cells and incubated for 10 min at 37°C. N-Phenylanthranilic acid (DPC) and gadolinium(III) chloride hexahydrate (Gd3+) were preincubated with cells for 2 h at 37°C, and 4-aminopyridine (4-AP) and tetraethylammonium chloride (TEA) were preincubated with cells for 1 h at 37°C. Unless indicated otherwise, all agents were dissolved in dimethyl sulfoxide (DMSO) and diluted in phosphate buffer prior to use (the final concentration of DMSO did not exceed 10%). DPC was dissolved in ethanol (5%), and Gd3+, 4-AP, and TEA were dissolved in water. Control cells were incubated in 10 mM phosphate buffer and vehicle (10% DMSO) with or without each compound. Following preincubation of cells with DIDS, NPPB, or niflumic acid, Hst 5 was added at a final concentration of 31 μM, and the preparations were incubated for an additional 1.5 h. The cell suspensions were diluted with phosphate buffer, and 500 cells were removed from each suspension and plated onto Sabouraud dextrose agar. Cell viability was assessed by counting the colonies that formed on plates after incubation for 24 h at 37°C. Concentrations of DIDS, NPPB, niflumic acid, and DMSO that did not affect cell viability were determined in preliminary experiments to avoid potential artifacts due to toxic effects on C. albicans cells. Candidacidal assays were performed in triplicate. Cell survival was expressed as follows: (number of colonies recovered from Hst 5-treated cells/number of colonies recovered from control cells) × 100. Alternatively, loss of viability was calculated as follows: [1 − (number of colonies recovered from Hst 5-treated cells/number of colonies recovered from control cells)] × 100. DIDS, NPPB, niflumic acid, TEA, 4-AP, and Gd3+ were obtained from Sigma; DPC was obtained from Alexic Corporation.
ATP bioluminescence assay.
ATP release from C. albicans cells was detected by a bioluminescence assay as previously described (14). Briefly, 106 cells in 10 mM sodium phosphate buffer (pH 7.4) were pretreated with 1 mM DIDS, 1 mM NPPB, or 1 mM niflumic acid for 2 h at 37°C; then Hst 5 was added at a final concentration of 31 μM, and the preparations were incubated for 30 min at 37°C with shaking. Control cultures were incubated with 10 mM PB buffer alone. For DIDS dose-response experiments, cells were pretreated with DIDS (0.1 to 1 mM) for 10 min at 37°C with shaking; this was followed by addition of Hst 5 to bring the final volume to 100 μl and incubation for 30 min at 37°C. The cells were pelleted, and 25 μl of each supernatant was added to 225 μl of boiling TE buffer (50 mM Tris, 2 mM EDTA; pH 7.8), boiled for 3 min, and stored on ice until it was assayed for ATP. A luciferin-luciferase assay mixture (100 μl) was added to 25 μl of cell supernatant in a 96-well microtiter plate, and light emission was monitored with a 1250 LKB-Wallac luminometer. Standard curves were produced for each experiment, and ATP concentrations were determined from these ATP standard curves. To eliminate the possibility that DIDS, NPPB, or niflumic acid had an effect on the bioluminescence reaction, each of the applied inhibitors was tested alone. No effect on luciferase enzyme activity was detected for any of these compounds. Data are presented below as means ± standard errors for at least three experiments. Student's t test was used to determine statistical significance between cell treatment groups.
Analysis of cell volume.
A Coulter Counter Multisizer II equipped with a Channelyzer ZM 256 instrument (Beckman Coulter, Miami, Fla.) with a 100-μm aperture was used to acquire mean cell volume measurements, and data were analyzed by using AccuComp software, version 4.1A. Strains were cultured as described above for the cell viability assays and used for measurements of cell volume. C. albicans cells (2 × 106 to 5 × 106 cells) were preincubated with 1 mM DIDS for 10 min before addition of 62 μM Hst 5 (a twofold higher concentration of Hst 5 was used to account for higher cell numbers). The cells were then added to 20 ml of isotonic NaCl buffer; measurements for 20,000 cells on average were determined and used to calculate the mean cell volume for each treatment and each time point. Cell volumes were measured at specified time points following addition of Hst 5 and compared with the initial values in isotonic buffer. Changes in the mean cell volume of Hst 5-treated cells were expressed as relative cell volumes compared to pretreatment values. Strains DB9 and DB13 grown for 12 h under inducing and noninducing conditions were diluted into isotonic NaCl buffer, and the mean cell volume for each population was measured directly. All of the experiments were carried out at room temperature.
Cell sorting and assessment of the viability of each cell population.
Single colonies of C. albicans DB9 and DB13 were inoculated into 5 ml of YNB medium containing 2% glucose and incubated at 37°C for 12 to 14 h with constant shaking at 250 rpm. Cells in the late log growth phase (100 μl containing 104 cells) were transferred into 100 ml of YNB medium containing 2% glucose (noninduced medium) or 2% sucrose (induced medium) and grown for another 24 h under the same conditions until the concentration reached 108 cells/ml. Cell pellets were harvested from each medium, washed twice with 10 mM sodium phosphate buffer (NaH2PO4-Na2HPO4; pH 7.2), and suspended in 5 ml of 10 mM sodium phosphate buffer. The cells were aspirated and ejected into a fluorescence-activated cell sorter (FACS) (Flow Cytometry Lab, Roswell Park Cancer Research Center, Buffalo, N.Y.). The heterogeneous populations from each induced strain were gated by size according to forward and side scatter intensity and sorted into small (R1) and large (R2) populations. The collected R1 population was the smallest 30% of the cells of both strains, and the collected R2 population was the largest 30% of the cells of both the DB9 and DB13 induced strains. The cell viability of each region was assessed by a dilution plate assay (7). The percentage of cell viability was calculated by dividing the number of cells that were able to grow on plates by the total number of cells collected for each region, as measured by the FACS. Cells (200 cells on average) from each region were examined microscopically, and the budding percentage was determined by dividing the number of cells with buds by the total number of cells and multiplying by 100.
DNA staining and DNA content analysis of sorted populations by flow cytometry.
DNA content was quantified by flow cytometry of the cells stained with the DNA-specific fluorescent dye Sytox Green (Molecular Probes, Inc.). The sorted populations (106 cells) of C. albicans strains DB9 and DB13 grown under induced and noninduced conditions were collected by centrifugation at 5,000 × g, washed twice with 1 ml of distilled H2O, and then fixed in 1 ml of cold 70% ethanol at 4°C. The cells were centrifuged at 1,000 × g and washed twice with sodium citrate buffer (50 mM C6H5Na3O7 · 2H2O) to rehydrate them, and then they were suspended in buffer containing 0.1 mg of RNase A (QIAGEN, Inc.) per ml and incubated at 37°C with constant shaking for 2 h. Sodium citrate buffer (0.5 ml) containing Sytox Green (final concentration,1 μM) was added to the cells, which were stained for 15 to 20 min in the dark. The DNA contents of each cell population were measured with a FACScan and were analyzed by using Modifit (Verity Software, Inc.). For time course experiments, single colonies of C. albicans DB9 were inoculated into 5 ml of YNB medium containing 2% glucose and incubated at 37°C for 12 to 14 h with constant shaking at 250 rpm. A total of 104 cells in the late log phase were transferred into 3 ml of YNB medium containing 2% glucose (noninducing medium) or 2% sucrose (inducing medium) and grown under the same conditions. The cell pellets were harvested at different times (0, 1, 2, 4, 7, 10, 13, and 23 h), washed twice with 1 ml of distilled H2O, and then fixed in 70% cold ethanol at 4°C. Cells (2 × 106 to 3 × 106 cells) were collected, washed twice with sodium citrate buffer, and processed as described above.
RNA isolation, cDNA synthesis, and RT-PCR analysis.
Isolation of total RNA from C. albicans strains DB9 and DB13 grown under inducing and noninducing conditions, control of DNA contamination, assessment of cDNA synthesis and efficiency, and reverse transcription (RT)-PCR analysis were performed essentially as reported previously (5). The following synthetic oligonucleotides were used: CLN1-5′ (5′-TAAGTGGATTAGATGAAGATTCAA-3′) and CLN1-3′ (5′-AACTTTATTGATGATATTGATGATG-3′) for CaCln1; CLN2-5′ (5′-GATTTGAGAAAAATTTGTGTTGAC-3′) and CLN2-3′ (5′-ATTGTTGAAGATAGAAAAGTGTAC-3′) for CaCln2; CYB1-5′ (5′-AGTACCACAACCAGAAGTAGG-3′) and CYB1-3′ (5′-TTTACTCTTCTGCTTCTGCTAC-3′) for CaCyb1; and CDC28-5′ (5′-ATGGTAGAGTTATCTGATTATCAA-3′) and CDC28-3′ (5′-TATTGCATGTTTTGGTGTTTGTC-3′) for CaCdc28. The sequences of these oligonucleotides were derived from previously published data (5, 23). All of these primer pairs amplify a fragment approximately 1 kb long, so the same PCR cycling parameters could be used for all four gene expression studies. The PCR cycling conditions were as follows: initial denaturation for 3 min at 94°C, followed by 30 cycles of 15 s of denaturation at 94°C, 15 s of annealing at 57°C, and 1 min of extension at 72°C and then final extension for 5 min at 72°C. All cDNA synthesis and RT-PCR experiments were performed at least three times and were highly reproducible.
RESULTS
Anion channel inhibitors protected C. albicans from Hst 5-induced cytotoxicity and ATP release.
We previously found that ATP release is an essential component of Hst 5-induced cytotoxicity for C. albicans and that chemical compounds that prevent ATP release also prevent cell death (14). DIDS is a broad-specificity chloride anion channel-blocking drug that has been shown to inhibit ATP release (20, 24) and cell death (18). Therefore, we investigated whether DIDS exhibited similar protective effects in C. albicans with respect to Hst 5-induced ATP release and cytotoxicity. Hst 5 added to C. albicans DS1 cells in our classical candidacidal assay system induced rapid release of ATP and caused killing of 100% of the cells. However, preincubation of C. albicans cells with 1 mM DIDS resulted in nearly total (94% ± 7%) inhibition of the cytotoxic effects and ATP release induced by 31 μM Hst 5 (Table 1). Protection from Hst 5 candidacidal activity was a function of DIDS concentration and time of preincubation, as C. albicans cells that were preincubated with 1 mM DIDS (the highest concentration tested) for 10 min were not sensitive to the lethal effects of Hst 5, while preincubation with 300 and 500 μM DIDS protected about 60% ± 21% and 82% ± 3%, respectively, of the cells from Hst 5 killing and preincubation with 100 μM DIDS for 10 min provided no protection from the lethal effects of 31 μM Hst 5. Although DIDS required less than 10 min of preincubation with cells to have its maximal effect, longer times of preincubation of cells with DIDS before addition of Hst 5 reduced its protective effect, showing that there was some reversibility of the DIDS interaction with yeast cells.
TABLE 1.
Loss of cell viability and ATP efflux from C. albicans cells pretreated with inhibitors
| Treatment | ATP efflux (pmol)a
|
Loss of cell viability (%)b
|
||
|---|---|---|---|---|
| Without Hst 5 | With Hst 5 | Without Hst 5 | With Hst 5 | |
| Buffer | 14 ± 6 | 1,168 ± 513 | 0 | 98 ± 3 |
| DIDS | 46 ± 3 | 88 ± 20c | 0 | 8 ± 7c |
| NPPB | 43 ± 15 | 227 ± 43c | 0 | 50 ± 6c |
| Niflumic acid | 20 ± 17 | 214 ± 76c | 0 | 49 ± 5c |
C. albicans cells were preincubated with DIDS (1 mM), NPPB (1 mM), niflumic acid (1 mM), or buffer alone for 2 h at 37°C, and then Hst 5 (31 μM) was added to cells for 30 min. The ATP released from 106 cells was quantified by using a luciferin-luciferase assay. The values are means ± standard deviations for at least three independent experiments.
C. albicans cells were preincubated with DIDS, NPPB, or niflumic acid (all at a concentration of 1 mM) for 10 min at 37°C and then treated with 31 μM Hst 5 for 1.5 h. Control cells were maintained in sodium phosphate buffer alone. Cells were plated after treatment to assess the loss of viability, determined as follows: [1 − (CFU of treated cells/CFU of control cells)] × 100. The values are means ± standard deviations for at least three independent experiments.
The difference between cells treated with Hst 5 alone and cells pretreated with inhibitor was significant at a P value of ≤0.01, as determined by a Student t test.
To determine whether these effects are specific to DIDS or whether other anion channel inhibitors have similar protective effects on Hst 5 lethality, we examined other pharmacological agents that inhibit anion channels in higher eukaryotic cells. Pretreatment of C. albicans cells with the anion channel-inhibiting compounds NPPB (1 mM) and niflumic acid (1 mM) resulted in significant protection of cells (50% ± 6% and 49% ± 5% cell killing, respectively) from Hst 5 (Table 1). However, DPC, a compound known to block cystic fibrosis Cl− channels, did not protect yeast cells from Hst 5 killing.
Because K+ release from C. albicans cells has been reported to be an early event following Hst treatment (30), we questioned whether compounds that inhibit the function of potassium channels could protect yeast cells from Hst 5 lethality. We tested several K+ channel inhibitors, including 4-PA (1 mM), which is a nonselective K+ channel inhibitor, and TEA (15 mM) and Gd3+ (1 mM), which are yeast potassium and stretch-activated cation channel inhibitors, respectively (10, 13). However, none of these inhibitors had any protective effect on Hst 5-induced killing of C. albicans cells (data not shown). Although it is possible that these compounds do not have pharmacological effects in yeast cells that are similar to those in higher eukaryotic cells, our classical candidacidal assay did not detect inhibition of Hst 5-mediated cell killing with cells pretreated with selected pharmacological inhibitors of cation channels.
Since incubation of C. albicans cells with Hst 5 induces ATP efflux from cells within 5 min and the efflux reaches maximal levels within 30 min (15), the abilities of DIDS, NPPB, and niflumic acid to prevent ATP release were evaluated. Cells preincubated with 1 mM DIDS and then treated with Hst 5 exhibited a more-than-10-fold reduction in ATP release (Table 1). Similarly, ATP release from Hst 5-treated C. albicans preincubated with NPPB (1 mM) and niflumic acid (1 mM) was reduced fivefold, and the protection corresponded to the attenuated protection from Hst 5-induced cell killing observed with DIDS. The reduction in ATP release from cells pretreated with DIDS (0.1 to 1 mM) prior to addition of Hst 5 was also linearly related to DIDS protection from cell killing (data not shown). These data suggest that Hst 5-induced ATP release occurs through a pathway sensitive to anion channel inhibitors.
Hst 5 induces cell volume loss that is inhibited by DIDS.
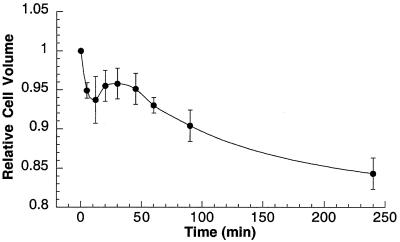
Since anion channels and ATP release have been implicated as regulators of cell volume homeostasis (16, 25), we examined whether Hst 5 itself could affect C. albicans cell volume. Addition of Hst 5 to cells resulted in an immediate loss of cell volume that could be detected within 5 min (the earliest measurable time point) following the addition (Fig. 1). After 12 min of incubation with Hst 5, the mean relative cell volume reached 0.937 ± 0.030, and it remained constant for 30 min of incubation with Hst 5. Then the mean cell volume continued to decrease, so that after 1.5 h (the standard time of incubation of Hst 5 with cells in the candidacidal assay), the mean cell volume decreased to 0.904 ± 0.020. The cells continued to shrink, reaching a relative mean volume of only 0.843 ± 0.020 compared with the basal cell volume 4 h after addition of Hst 5.
FIG. 1.
Time-dependent reduction in mean cell volume of C. albicans cells induced by exogenous addition of Hst 5. C. albicans DS1 cells (2 × 106 to 5 × 106 cells) were treated with Hst 5 in 10 mM sodium phosphate buffer (pH 7.4) at room temperature and incubated for different times. The mean cell volume (average for 20,000 cells) was measured with a Coulter Counter Multisizer II-Channelyzer at different times following addition of Hst 5 and compared with the initial value. The data was analyzed by using AccuComp software, and the changes in mean cell volume are expressed as relative cell volumes compared to the pretreatment values. The data are means ± standard deviations for three independent observations.
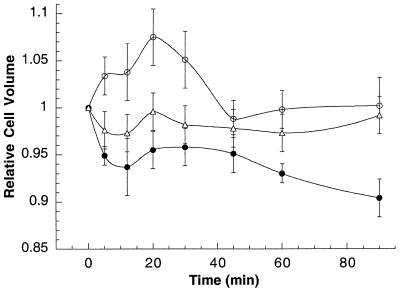
We investigated whether DIDS could also block the yeast cell volume decrease as a result of Hst 5 treatment (Fig. 2). C. albicans cells maintained in sodium phosphate buffer (10 mM, pH 7.4) (data not shown) or treated with 1 mM DIDS alone (Fig. 2) exhibited no change in cell volume throughout the 90-min experiment (mean cell volume, 0.978 ± 0.02). However, cells pretreated with 1 mM DIDS for 10 min prior to Hst 5 addition exhibited an immediate cell volume increase upon Hst 5 addition (Fig. 2). After 20 min of exposure to Hst 5, the mean cell volume increased to a maximum of 1.075 ± 0.030, after which the volume returned to pretreatment levels by 45 min. Subsequently, the mean cell volume remained constant at 1.002 ± 0.030 despite continued incubation with Hst 5. These data show that preincubation of C. albicans cells with DIDS completely eliminated the cell volume decrease induced by Hst 5 and suggested that the protection from Hst 5-induced yeast cell death provided by DIDS may be due to elimination of the cell volume decrease.
FIG. 2.
Hst 5-induced cell volume change is inhibited by pretreatment with DIDS. C. albicans DS1 cells (5 × 106 cells) were preincubated with 1 mM DIDS for 10 min before addition of 62 μM Hst 5 in 10 mM phosphate buffer at room temperature (○). Cells were also treated only with Hst 5 (•). Control cells were preincubated with 1 mM DIDS alone (▵). The mean cell volume for each treatment and each time point was measured with a Coulter Counter Multisizer II-Channelyzer, and cell volume changes are expressed as relative cell volumes compared to pretreatment values (zero time). All of experiments were carried out at room temperature. The data are means ± standard deviations for three independent experiments.
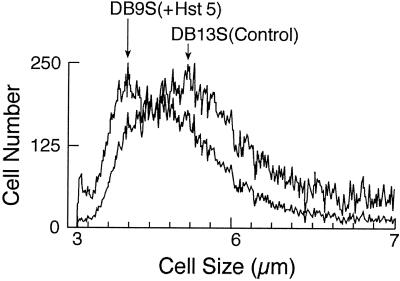
C. albicans DB9 cell cultures exhibited 50 to 60% reductions in population growth 23 h after induction of Hst 5 expression (1). To determine whether the cell volumes also were reduced, we measured changes in the mean cell volumes of the populations. We noted that a change in the growth medium from a glucose (noninducing) medium to a sucrose (inducing) medium itself caused a reduction in the mean cell volume. Therefore, C. albicans DB13 cells that express nontoxic statherin (a salivary protein whose size is similar to that of Hst 5) under sucrose induction conditions were utilized as control cells in an assessment of volume changes related to specific protein expression. C. albicans DB13 control cells that express statherin (23 h) had a relative mean cell volume of 0.94 ± 0.02 compared with the volume of noninduced cells. However, 23 h following Hst 5 induction C. albicans cells had a significantly reduced relative mean cell volume (0.82 ± 0.02) compared with the volume of cells expressing statherin. Overlay profiles representing the population mean cell diameters of cells expressing Hst 5 (DB9) or statherin (DB13) from one representative experiment are shown in Fig. 3.
FIG. 3.
Induction of Hst 5 expression in C. albicans DB9 cells causes reduction in mean cell size. C. albicans DB9 and DB13 cells were grown for 23 h in sucrose to induce expression of Hst 5 (DB9) and a nontoxic control protein, statherin (DB13). Cells were collected, washed, and placed into isotonic NaCl buffer, and the cell volume for each population was measured with a Coulter Counter Multisizer II-Channelyzer. Cell size is expressed as the cell diameter. The overlay profile shows the reduction in mean cell size of DB9 cells expressing Hst 5 (left arrow) compared with the mean cell size of DB13 cells expressing statherin (right arrow).
Since DIDS was found to be highly effective in protecting cells from Hst 5-induced lethality in the conventional candidacidal assay, we sought to test this compound with cells expressing Hst 5. Under the static conditions of the classical candidicidal assay, protection from Hst 5 killing by DIDS is reduced with increasing (>30 min) time of preincubation with cells. Since the lethal effects of Hst 5 in induced cells are first noted about 1 h following induction of the cells (with this preincubation time 1 mM DIDS has a 50% protective effect), we could examine DIDS protection only at early times of Hst 5 expression with the expectation that DIDS would provide about 50% protection from lethal effects. At 1 h following initiation of Hst 5 expression, cell culture growth was reduced by 26.1% ± 5.1% compared with the growth of control cultures, and by 2 h following induction, cell growth was reduced by 29.3% ± 5.4% (n = 3). Addition of 1 mM DIDS to these induced cultures resulted in cell growth that was reduced by only 6.91% ± 1.2% and 13.9% ± 1.6% (n = 3) at 1 and 2 h, respectively, following induction of Hst 5. These results show that DIDS provided more than 50% protection from cell death in cells with induced expression of Hst 5 compared to the data obtained for the classical candidacidal system. Overall, these findings support the premise that Hst 5 exerts lethal effects in C. albicans cells through cell volume changes that are inhibited by DIDS and that this effect is observed both in cells with exogenously added Hst 5 (classical system) and in cells expressing intracellular Hst 5, suggesting that the mechanisms of Hst 5 activity in these two systems are similar.
Expression of intracellular Hst 5 results in an increase in the percentage of small cells in the G1 phase.
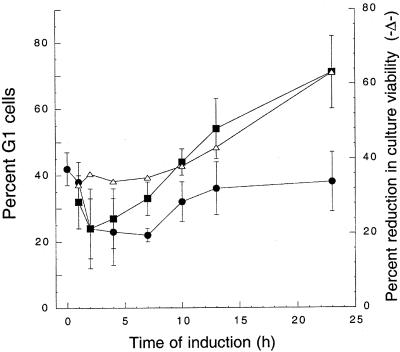
Budding yeast must attain a critical cell size in order to progress past the G1 phase; thus, Hst 5-induced volume loss and/or disruption of volume regulation may prevent progression of Candida cells past a cell size checkpoint, leading to G1 cell cycle arrest. To investigate this possibility, we measured the percentage of G1 cells over 23 h following induction of expression of Hst 5. Early-stationary-phase cells were divided and grown to the late logarithmic phase (23 h) under inducing (Hst 5) and noninducing asynchronous growth conditions. Analysis of the noninduced cells (DB9) showed that there was a reduction in the percentage of G1 phase cells (from 42% ± 5% to 22% ± 2% at 7 h) and a corresponding increase in the percentage of cells in the G2/M and S phases as the population reached the mid-log growth phase (Fig. 4). Subsequently, the percentage of noninduced cells (without Hst 5) in the G1 phase gradually increased to 38% ± 9% by 23 h. In contrast, the percentage of cells in the G1 phase in the induced culture (DB9S) expressing Hst 5 remained nearly constant over the initial 7 h and then steadily increased to 44% ± 4%, 54% ± 9%, and 71% ± 11% at 10, 13, and 23 h, respectively, following induction of Hst 5 (Fig. 4). The increase in the percentage of G1 cells paralleled the loss of the ability to replicate in Hst 5-induced cultures (Fig. 4), showing that the candidacidal effects of Hst 5 are accompanied by an increase in the percentage of G1 cells typical of cell cycle arrest.
FIG. 4.
Induction of Hst 5 expression in C. albicans DB9 cells results in an increased percentage of G1 cells. DB9 cells were assayed at different times following inoculation into cultures containing glucose (without Hst 5) (•) or sucrose (inducing Hst 5) (▪). Cells were stained with Sytox Green, and the DNA content for the total DB9 cell population under inducing (▪) and noninducing (•) conditions was measured and analyzed with the FACScan by using Modifit software (Verity Software, Inc.) to determine the percentage of G1 cells. The viability of DB9 cells expressing Hst 5 was measured by a dilution plate assay at each time point (▵).
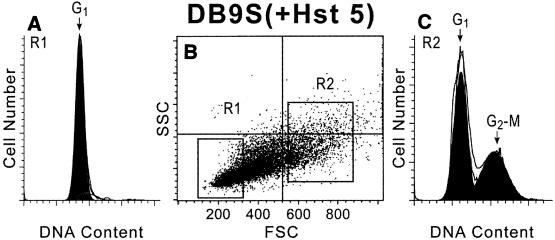
Since growth reduction following expression of Hst 5 in cell cultures is accompanied by an overall increase in the percentage of G1 cells and a reduction in the total mean cell volume, we questioned whether smaller cells in this population also contained a higher percentage of G1 cells. Our strain expressing Hst 5 was well suited for these experiments since the asynchronous cultures contained a heterogeneous population of growing and nonviable cells as a result of continuous Hst 5 expression. We also compared subpopulations of large and small cells obtained by gated cell sorting both from induced cultures expressing Hst 5 and from cultures expressing the nontoxic protein statherin following 23 h of growth. Regions were gated to collect the largest (R2) and smallest (R1) 30% of each population for analysis. Analysis of FACS profiles of cells with expressed Hst 5 showed that the percentages of G1 cells (1N DNA content) were 58% for the large R2 group (Fig. 5C) and 98% for the small R1 cells (Fig. 5A). Control cells expressing statherin were similarly examined by size, and the percentages of G1 cells were 45% for the large R2 group and 86% for the small R1 cells (data not shown). To further assess the characteristics of these subpopulations, cells were collected from each group and assessed for viability by plating, and the percentage of budded cells was examined. Large R2 cells from cultures expressing either control protein or Hst 5 were found to be mostly (94 to 97%) viable. Analysis of the smallest 30% of cells (R1) of the statherin-expressing strain showed that 58% of the small cells were viable, a value similar to the value obtained for the subpopulation of the smallest 30% of noninduced cells (54%). Thus, both statherin-expressing and noninduced DB9 cell cultures contained a subpopulation of small cells that did not replicate upon plating and may have represented replicative senescent cells. In contrast, only 35% of the small R1 cells from Hst 5-expressing cultures were viable upon plating. The percentage of budded cells in Hst 5-induced cells was 34.5% for the large R2 cells but only 3.8% for the small R1 cells, indicating that loss of viability in small cells is also associated with a reduction in the number of budded cells. Altogether, these data suggest that induction of Hst 5 causes a reduction in the mean population cell volume that is coupled with an increase in the percentage of G1 phase cells, so that nearly all of the smallest R1 cells from Hst 5-induced cultures are in the G1 phase and are unbudded.
FIG. 5.
Small cells sorted following Hst 5 expression in C. albicans DB9 cells are solely in the G1 phase with a 1N DNA content. C. albicans DB9 cells were grown in medium containing 2% sucrose (inducing medium) for 23 h, washed, and measured by using FACS. The cells were gated by size according to forward scatter (FSC) and side scatter (SSC) intensity (gates indicated in panel B) and sorted according to the smallest 30% (R1) (A) and largest 30% (R2) (C) of the cells. Collected cells from the R1 and R2 regions were analyzed for DNA content by flow cytometry following fixation and staining with Sytox Green. Analysis of the G1 and G2-M phase contents (1N and 2N DNA contents, respectively) was done by using Modifit software. Data from one experiment are shown, and similar results were observed in repeated experiments.
Inducible expression of Hst 5 results in reduced Cln1 and Cln2 transcript levels.
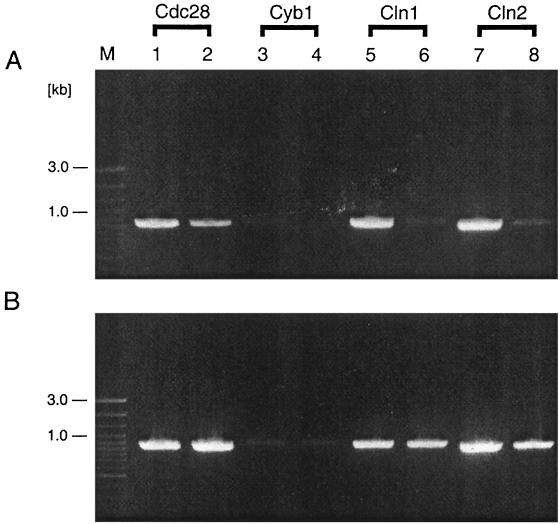
Because significant numbers of induced DB9 cells expressing Hst 5 accumulated in the G1 phase, we examined the levels of specific G1 transcripts in the populations of induced and noninduced DB9 cells and compared these levels to the levels in the statherin-expressing DB13 strain. For these experiments total RNA from the entire populations of exponentially growing induced and noninduced DB9 (Hst 5-expressing) and DB13 (statherin-expressing) cells were isolated and subjected to RT-PCR analysis. As shown in Fig. 6, Cln1 and Cln2 transcripts were present at the same levels in the noninduced DB9 and DB13 and the induced DB13 cell populations. In contrast, the levels of these transcripts were greatly reduced in the Hst 5-expressing DB9 cell cultures, which correlated with the G1 arrest data observed previously. These experiments demonstrate that the defect in G1 cyclin expression at the transcriptional level may also be the result of Hst 5 candidacidal action. In addition, the RNA samples were analyzed for the transcript of the mitotic cyclin CYB1, which is thought to be the C. albicans counterpart of Saccharomyces cerevisiae CLB1/CLB2 (5). There was no difference in the levels of this B-type cyclin in the induced and noninduced cell populations studied (Fig. 6). In addition, we performed an RT-PCR analysis of the transcription of Cdc28, which in both budding and fission yeasts is involved in G1-S and G2-M transitions (5). Again, no significant transcript level differences were observed (Fig. 6). The results of these additional experiments, combined with the results of the DNA content measurement experiments described above, argue against disturbances in cell mitosis and support the G1 arrest hypothesis.
FIG. 6.
Hst 5 expression results in reduced Cln1 and Cln2 transcript levels. Analysis of the RT-PCR products in total RNA samples obtained from C. albicans strain DB9 (Hst 5-expressing) (A) and DB13 (statherin-expressing) (B) cells was done to examine the levels of specific G1 cyclin transcripts. Lanes 1, 3, 5, and 7, RNA samples obtained from cultures grown on YNB-glucose (noninducing conditions); lanes 2, 4, 6, and 8, RNA samples obtained from cultures grown on YNB-sucrose (inducing conditions). The designations of the C. albicans genes examined are indicated at the top. Lane M contained molecular mass markers.
DISCUSSION
ATP release and K+ efflux have been observed to be consistent aspects of Hst 5-induced killing of C. albicans (14, 30). ATP efflux was correlated with cell death, and chemical compounds that inhibited ATP efflux also partially prevented cell death (15). In this study, we showed that inhibitors of anion transporters or volume-regulating Cl− channels, including DIDS, NPPB, and niflumic acid, provided complete or substantial protection from Hst 5-induced killing of C. albicans. In each instance, ATP release was also reduced parallel to inhibition of fungicidal activity. This effect was specific for anion channel inhibitors, as a nonselective K+ channel inhibitor (4-PA), as well as TEA and Gd3+ (both nonselective cation channel inhibitors), were ineffective in preventing Hst 5 candidacidal activity.
Recently, evidence of noncytolytic ATP release across the plasma membrane has been obtained for many cell types. Although ATP channels have not been identified, mechanical stimuli and osmotic cell swelling are both ubiquitous inducers of noncytolytic ATP release (11, 24, 27). Our finding that lethal concentrations of Hst 5 induced rapid volume loss and ATP efflux in C. albicans cells that were completely eliminated by pretreatment of cells with DIDS suggests that anion transporters are key components in volume regulation involved in Hst 5 killing of yeast.
Yeast membrane transporters have a well-defined role in cell volume regulation. Cells decrease their volume by efflux of Cl− and K+ ions, with concomitant movement of water out of the cells. Rapid efflux of potassium and magnesium from C. albicans cells following incubation with Hst occurs (30), suggesting that ion movement from Hst-treated yeast cells is an initial event in Hst killing activity. Our data showing that there is rapid volume loss following Hst 5 treatment of cells suggests that Hst 5 may activate transport of anions parallel to efflux of K+ and water from C. albicans. The protection against Hst 5-induced volume reduction provided by DIDS may be a result of DIDS inhibition of outward Cl− efflux or some other more generalized anion transport that prevents cell volume decrease. Interestingly, we observed that cells pretreated with DIDS before addition of Hst 5 showed an initial increase in cell volume before recovery to basal levels by 45 min (Fig. 2). These data suggest that Hst 5 affects other ion channels in addition to those blocked by DIDS but that these effects are reversible and nonlethal when DIDS-inhibitable channels or transporters are blocked.
The data point towards crucial involvement of Hst 5-initiated ion flux in C. albicans and imply that the extracellular ionic medium may have significant effects on Hst 5 fungicidal activity. Although saliva is a hypotonic fluid compared with blood, the levels of some ions, such as phosphate and potassium, are 5 to 10 times higher in saliva than in plasma (8). Individual electrolyte concentrations in saliva also vary widely as functions of the salivary flow rate, age, systemic diseases, and use of pharmacological agents (8). Therefore, changes in the ionic environment in saliva modulated by host salivary flow rates or other factors may protect yeast from Hst 5 killing and may be a key factor in initiation of oropharyngeal candidiasis. The alterations in salivary ionic composition could be the reason for reports of patients with oral candidiasis despite normal or elevated levels of salivary Hsts (2).
Since Hst 5-induced C. albicans cell volume loss is correlated with cell death in our system, we questioned how cell volume reduction and ATP release contribute to the lethal effects of Hst 5. The G1 stage is a critical point in the progression of yeast through the cell cycle. In budding yeast, cells in the G1 phase do not initiate a new round of cell division unless certain conditions, such as the availability of nutrients and a critical cell size/volume ratio, are met. At a critical size, cells progress through Start (also referred to as the Restriction point), which commits them to a new round of cell division (9, 22). Passage through Start is characterized by production of G1 cyclins, which activate cyclin-dependent kinase activity and thereby initiate the events associated with the subsequent S phase and mitosis (9, 29). Growth of strain DB9 producing Hst 5 in inducing conditions caused an accumulation of cells in G1 with 1N DNA and greatly reduced the levels of Cln1 and Cln2 mRNA, suggesting that these cells were arrested before Start. In contrast to S. cerevisiae, in which cells continue to increase in size although they are arrested in G1 by loss of function of CDC28 or G1 cyclins (4), C. albicans DB9 cells that are arrested due to intracellular expression of Hst 5 remain small. In this regard, these cells appear to be more similar to the S. cerevisiae cells observed with G1 arrest caused by nutrient deprivation (28) or by mutations in Cdc25 and Cdc35 that cause arrest before Start without an increase in cell size (3, 6). The cellular release of ATP that is typically associated with Hst 5 candidacidal activity also may result in an inability of the organism to import nutrients and in cellular nutrient depletion, thereby maintaining a small cell size and G1 arrest.
The finding that the transcript levels of CaCdc28 and CaCyb1 do not appear to be affected significantly upon induction of Hst 5 in DB9 cells suggests that the transcription of G1 cyclin genes might be specifically down regulated in these cells. These results also suggest that the observed cell cycle arrest is unlikely to be due to general transcription and, possibly, translation machinery defects caused by Hst 5 action. Therefore, it appears that the observed G1 arrest is a consequence of Hst 5 disturbance of the cell volume/size ratio and the loss of ATP, leading to an inability of the targeted cells to reach a critical mass that is a prerequisite for commitment to a new round of cell division. Alternatively, the initial volume reduction in Hst 5-treated cells could lead to G1 arrest that subsequently leads to further size reduction. A pause in overall cell volume reduction around 40 to 60 min after Hst 5 treatment was observed (Fig. 1), which was then followed by further cell size reduction. This biphasic response is consistent with a postulated dual sequence of events. Further experiments are needed to determine whether cell size reduction or down regulation of G1 cyclins is the ultimate cause of the observed G1 arrest.
Our data suggest that a key determinant in the mechanism of Hst 5 toxicity in C. albicans cells is disruption of the regulatory circuits for ion transport and cell volume homeostasis. These processes appear to be closely coupled with loss of intracellular ATP and may also be accompanied by loss of cellular amino acids or ions. Ultimately, these Hst 5-induced disturbances of cell volume regulation and ion transport may lead to G1 arrest of cells that resembles nutrient starvation cell cycle arrest in S. cerevisiae. This novel process of fungicidal activity by a human salivary protein has highlighted potential interactions of Hst 5 with volume regulatory mechanisms and the process of yeast cell cycle control.
Acknowledgments
This work was supported by Public Health Service grants DE10641 and DE00406 from the National Institute of Dental and Craniofacial Research (to M.E.).
We thank Svetlana Koshlukova for her critical reading of the manuscript and for helpful scientific comments.
Editor: R. N. Moore
REFERENCES
- 1.Baev, D., X. Li, and M. Edgerton. 2001. Genetically engineered human salivary histatin genes are functional in Candida albicans: development of a new system for studying histatin candidacidal activity. Microbiology 147:3323-3334. [DOI] [PubMed] [Google Scholar]
- 2.Bercier, J. G., I. Al-Hashimi, N. Haghighat, T. D. Rees, and F. G. Oppenheim. 1999. Salivary histatins in patients with recurrent oral candidiasis. J. Oral Pathol. Med. 28:26-29. [DOI] [PubMed] [Google Scholar]
- 3.Broek, D., T. Toda, T. Michaeli, L. Levin, C. Birchmeier, M. Zoller, S. Powers, and M. Wigler. 1987. The S. cerevisiae CDC25 gene product regulates the RAS/adenylate cyclase pathway. Cell 48:789-799. [DOI] [PubMed] [Google Scholar]
- 4.Cross, F. 1990. Cell cycle arrest caused by CLN gene deficiency in Saccharomyces cerevisiae resembles START-I arrest and is independent of the mating-pheromone signalling pathway. Mol. Cell. Biol. 10:6482-6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damagnez, V., and G. Cottarel. 1996. Candida albicans CDK1 and CYB1: cDNA homologues of the cdc2/CDC28 and cdc13/CLB1/CLB2 cell cycle control genes. Gene 172:137-141. [DOI] [PubMed] [Google Scholar]
- 6.Dawes, I., and G. Calvert. 1984. Initiation of sporulation in Saccharomyces cerevisiae. Mutations causing derepressed sporulation and G1 arrest in the cell division cycle. J. Gen. Microbiol. 130:605-613. [DOI] [PubMed] [Google Scholar]
- 7.Edgerton, M., S. E. Koshlukova, T. E. Lo, B. G. Chrzan, R. M. Straubinger, and P. A. Raj. 1998. Candidacidal activity of salivary histatins. Identification of a histatin 5-binding protein on Candida albicans. J. Biol. Chem. 273:20438-20447. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson, D. B. 1989. Salivary electrolytes, p. 75-100. In J. O. Tenovuo (ed.), Human saliva: clinical chemistry and microbiology, vol. 1. CRC Press, Boca Raton, Fla.
- 9.Futcher, B. 1996. Cyclins and the wiring of the yeast cell cycle. Yeast 12:1635-1646. [DOI] [PubMed] [Google Scholar]
- 10.Gustin, M. C., B. Martinac, Y. Saimi, M. R. Culbertson, and C. Kung. 1986. Ion channels in yeast. Science 233:1195-1197. [DOI] [PubMed] [Google Scholar]
- 11.Hazama, A., A. Miwa, T. Miyoshi, T. Shimizu, and Y. Okada. 1998. Cell volume regulation: the molecular mechanism and volume sensing machinery, p. 93-98. In Y. Okada (ed.), Cell volume regulation. Elsevier, Amsterdam, The Netherlands.
- 12.Helmerhorst, E. J., P. Breeuwer, W. Van't Hof, E. Walgreen-Weterings, L. C. Oomen, E. C. Veerman, A. V. Amerongen, and T. Abee. 1999. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J. Biol. Chem. 274:7286-7291. [DOI] [PubMed] [Google Scholar]
- 13.Kanzaki, M., M. Nagasawa, I. Kojima, C. Sato, K. Naruse, M. Sokabe, and H. Iida. 1999. Molecular identification of a eukaryotic, stretch-activated nonselective cation channel. Science 285:882-886. [DOI] [PubMed] [Google Scholar]
- 14.Koshlukova, S. E., T. L. Lloyd, M. W. B. Araujo, and M. Edgerton. 1999. Salivary histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. J. Biol. Chem. 274:18872-18879. [DOI] [PubMed] [Google Scholar]
- 15.Koshlukova, S. E., M. W. B. Araujo, D. Baev, and M. Edgerton. 2000. Released ATP is an extracellular cytotoxic mediator in salivary histatin 5-induced killing of Candida albicans. Infect. Immun. 68:6848-6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang, F., G. L. Busch, and H. Volkl. 1998. The diversity of volume regulatory mechanisms. Cell. Physiol. Biochem. 8:1-45. [DOI] [PubMed] [Google Scholar]
- 17.Lin, A. L., Q. Shi, D. A. Johnson, T. F. Patterson, M. G. Rinaldi, and C.-K. Yeh. 1999. Further characterization of human salivary anticandidal activities in a human immunodeficiency virus-positive cohort by use of microassays. Clin. Diagn. Lab. Immunol. 6:851-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maeno, E., Y. Ishizaki, T. Kanaseki, A. Hazama, and Y. Okada. 2000. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc. Natl. Acad. Sci. USA 97:9487-9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okada, Y. 1997. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am. J. Physiol. 273:C755-C789. [DOI] [PubMed] [Google Scholar]
- 20.Okada, Y., E. Maeno, T. Shimizu, K. Dezaki, J. Wang, and S. Morishima. 2001. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J. Physiol. 532:3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oppenheim, F. G., T. Xu, F. M. McMillian, S. M. Levitz, R. D. Diamond, G. D. Offner, and R. F. Troxler. 1988. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 263:7472-7477. [PubMed] [Google Scholar]
- 22.Planas-Silva, M. D., and R. A. Weinberg. 1997. The restriction point and control of cell proliferation. Curr. Opin. Cell Biol. 9:768-772. [DOI] [PubMed] [Google Scholar]
- 23.Sherlock, G., A. M. Bahman, A. Mahal, J.-C. Shieh, M. Ferreira, and J. Rosamond. 1994. Molecular cloning and analysis of CDC28 and cyclin homologues from the human fungal pathogen Candida albicans. Mol. Gen. Genet. 245:716-723. [DOI] [PubMed] [Google Scholar]
- 24.Sprague, R. S., M. L. Ellsworth, A. H. Stephenson, M. E. Kleinhenz, and A. J. Lonigro. 1998. Deformation-induced ATP release from red blood cells requires CFTR activity. Am. J. Physiol. 275:H1726-H1732. [DOI] [PubMed] [Google Scholar]
- 25.Strange, K., F. Emma, and P. S. Jackson. 1996. Cellular and molecular physiology of volume-sensitive anion channels. Am. J. Physiol. 270:C711-C730. [DOI] [PubMed] [Google Scholar]
- 26.Wang, Y., R. Roman, S. D. Lidofsky, and J. G. Fitz. 1996. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc. Natl. Acad. Sci. USA 93:12020-12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watt, W. C., E. R. Lazarowski, and R. C. Boucher. 1998. Cystic fibrosis transmembrane regulator-independent release of ATP. Its implications for the regulation of P2Y2 receptors in airway epithelia. J. Biol. Chem. 273:14053-14058. [DOI] [PubMed] [Google Scholar]
- 28.Werner-Washburne, M., E. Braun, G. Johnston, and R. Singer. 1993. Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol. Rev. 57:383-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wittenberg, C., K. Sugimoto, and S. I. Reed. 1990. G1-specific cyclins of S. cerevisiae: cell cycle periodicity, regulation by mating pheromone, and association with the p34CDC28 protein kinase. Cell 62:225-237. [DOI] [PubMed] [Google Scholar]
- 30.Xu, Y., I. Ambukar, H. Yamagishi, W. Swaim, T. Walsh, and B. C. O'Connell. 1999. Histatin 3-mediated killing of Candida albicans: effect of extracellular salt concentration on binding and internalization. Antimicrob. Agents Chemother. 43:2256-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]