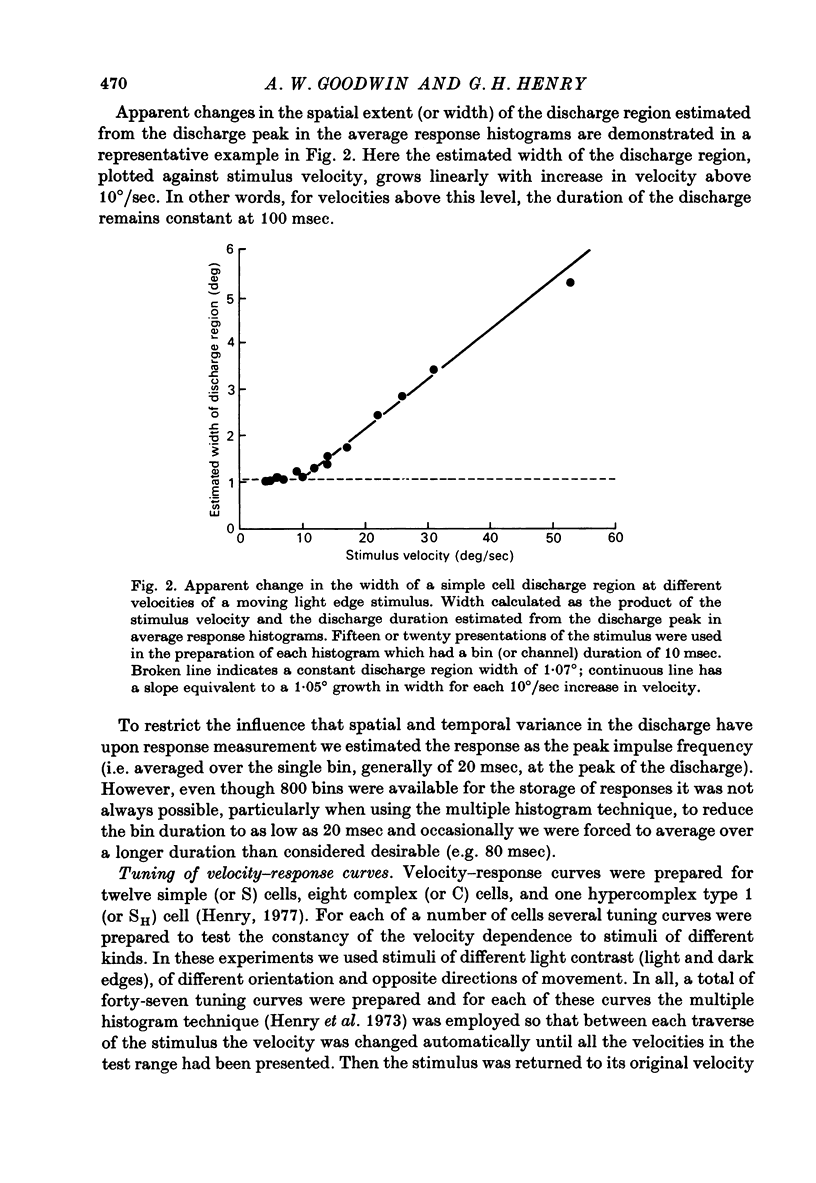
Abstract
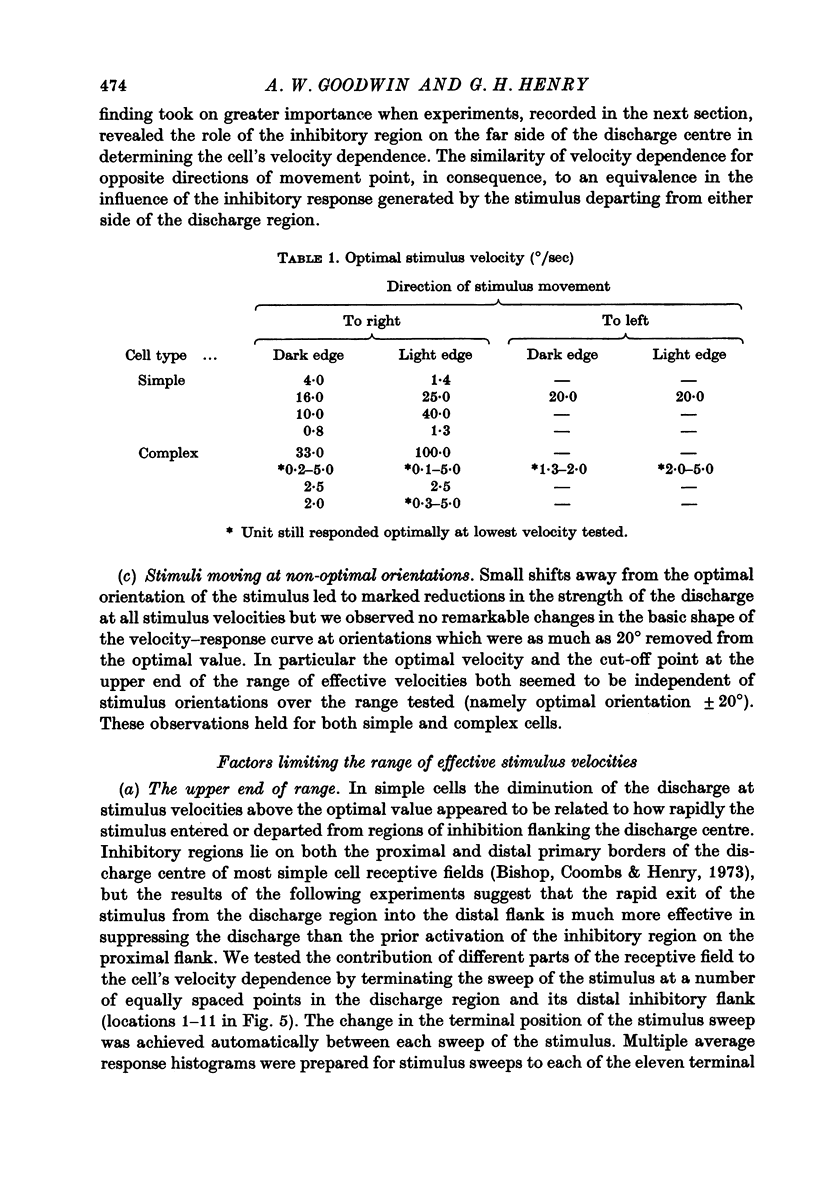
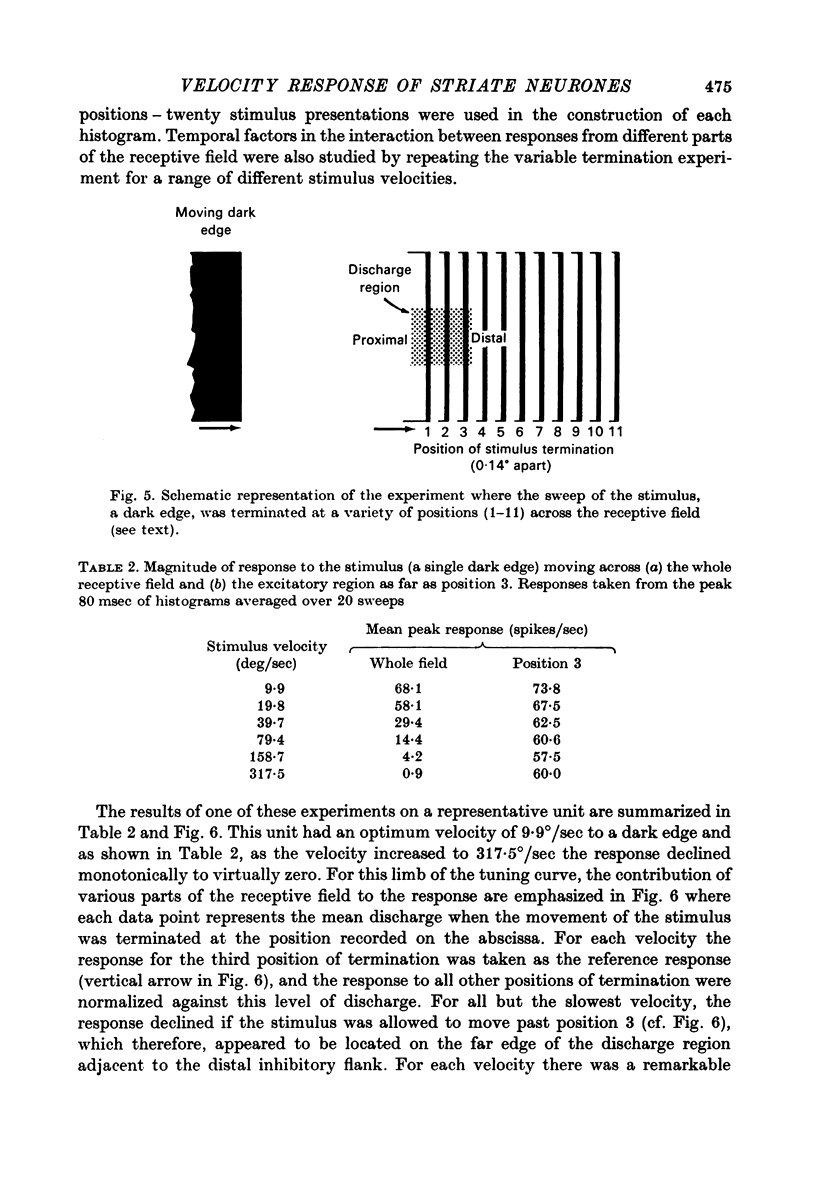
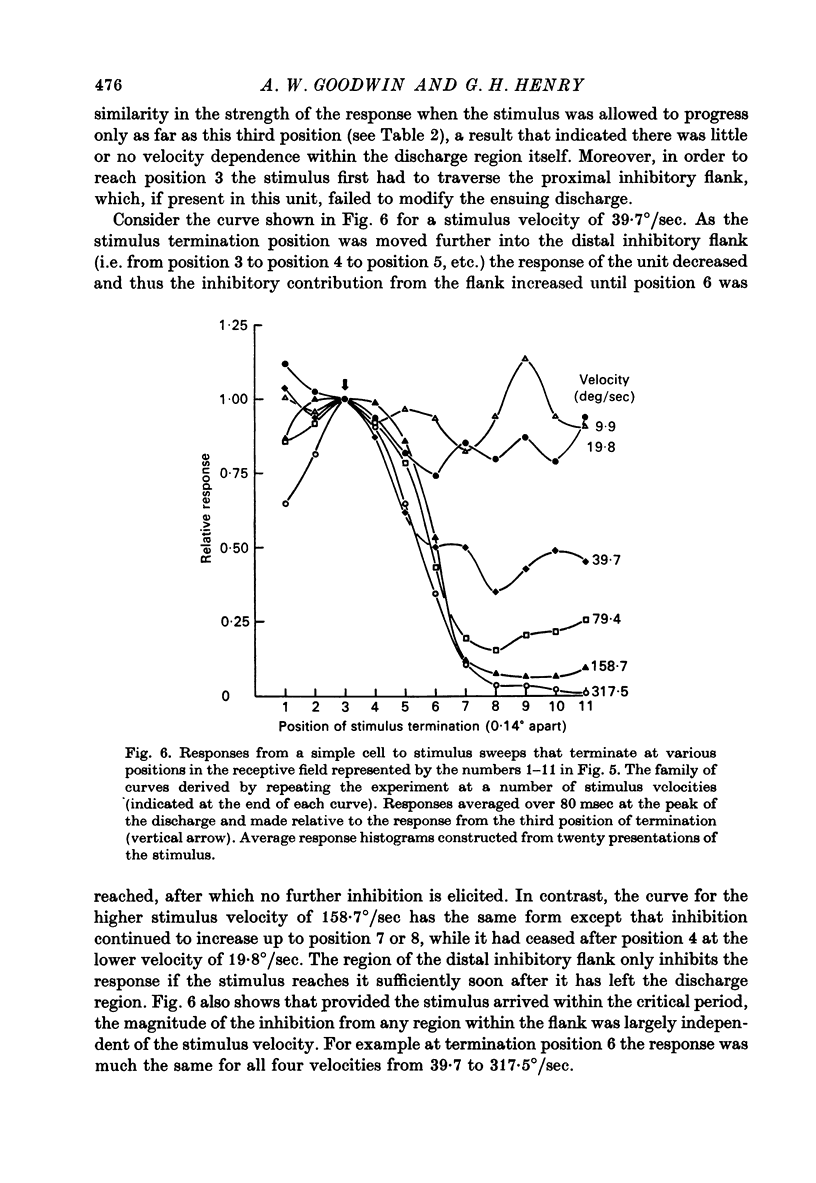
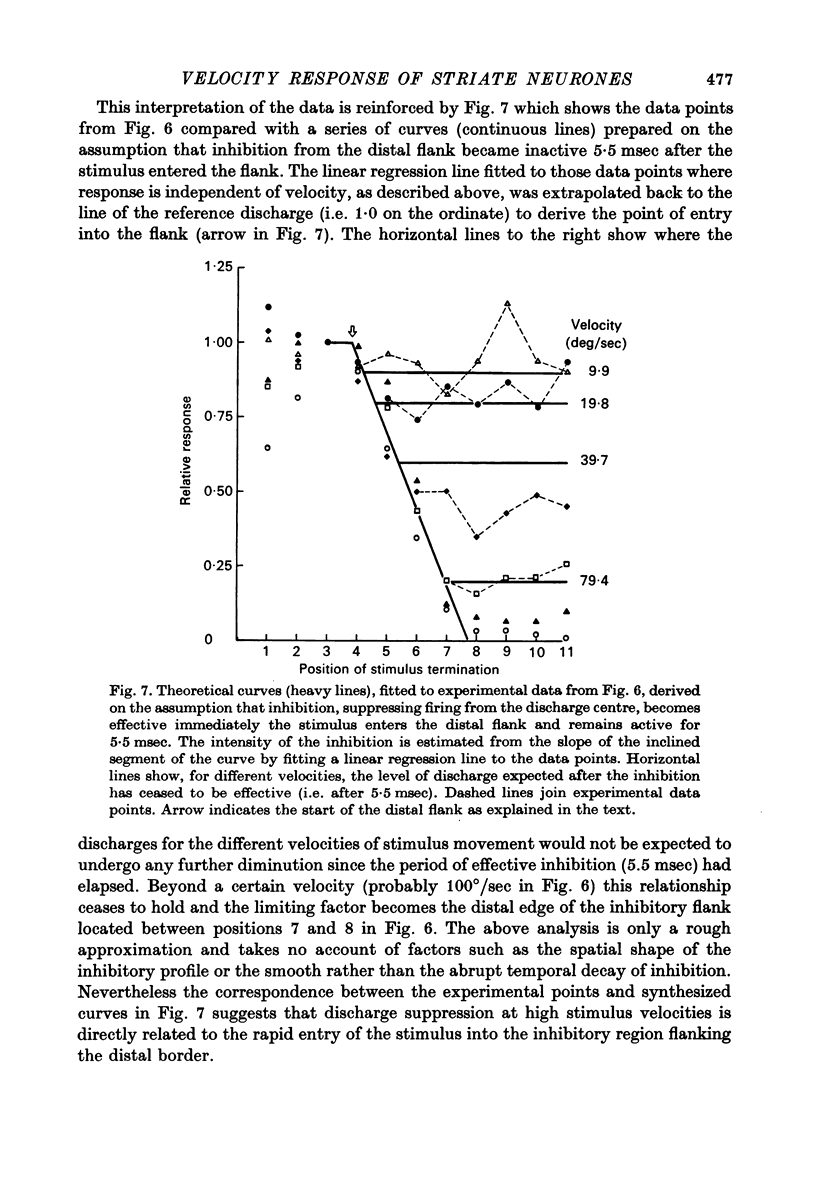
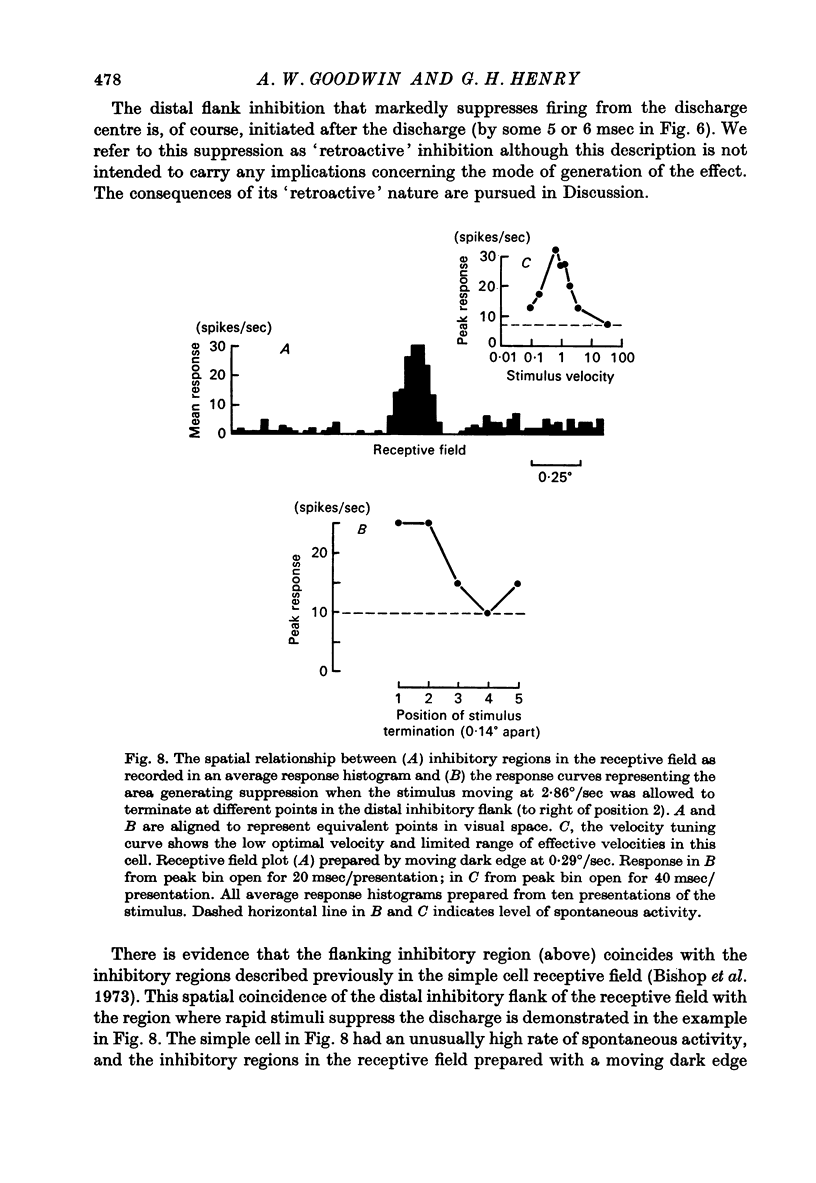
1. Using a multi-histogram technique forty-seven response-velocity curves were prepared for a variety of visual stimuli presented to twenty-one cells in the striate cortex of the anaesthetized, paralysed cat. 2. The character of each velocity-response curve varied according to the measurement used in assessing a response. Reasons are advanced for sampling the response over a single bin of short duration at the peak of the discharge in each average response histogram. 3. The sharpness of tuning varied markedly throughout the population of cells but it was not possible to establish any definitive class differences. 4. For simple and complex cell categories there was considerable overlap in both the range of effective stimulus velocities and the distribution of the optimal velocities. An observation not emphasized in the past was that some simple cells responded to very fast stimuli while a number of complex cells were driven by very slowly moving stimuli. 5. Generally changes in stimulus parameters such as the polarity of contrast of a moving edge, its orientation or direction of movement produced only slight modifications in the profile of the velocity-response curve. 6. The abolition of the response of simple cells that failed to be driven by rapidly moving stimuli was shown to be due to the entry of the stimulus into the inhibitory flank distal to the discharge region. When the movement of the stimulus was confined to the discharge region there was little evidence of velocity dependence in the response. The duration over which the inhibition from the distal flank remained effective was evaluated for representative simple cells.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop P. O., Coombs J. S., Henry G. H. Receptive fields of simple cells in the cat striate cortex. J Physiol. 1973 May;231(1):31–60. doi: 10.1113/jphysiol.1973.sp010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Coombs J. S., Henry G. H. Responses to visual contours: spatio-temporal aspects of excitation in the receptive fields of simple striate neurones. J Physiol. 1971 Dec;219(3):625–657. doi: 10.1113/jphysiol.1971.sp009681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher B., Sanderson K. J. Receptive field analysis: responses to moving visual contours by single lateral geniculate neurones in the cat. J Physiol. 1973 Oct;234(1):95–118. doi: 10.1113/jphysiol.1973.sp010336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin A. W., Henry G. H. Direction selectivity of complex cells in a comparison with simple cells. J Neurophysiol. 1975 Nov;38(6):1524–1540. doi: 10.1152/jn.1975.38.6.1524. [DOI] [PubMed] [Google Scholar]
- HICK W. E. The threshold for sudden changes in the velocity of a seen object. Q J Exp Psychol. 1950 Feb;2(1):33–41. doi: 10.1080/17470215008416572. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O., Tupper R. M., Dreher B. Orientation specificity and response variability of cells in the striate cortex. Vision Res. 1973 Sep;13(9):1771–1779. doi: 10.1016/0042-6989(73)90094-1. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Dreher B., Bishop P. O. Orientation specificity of cells in cat striate cortex. J Neurophysiol. 1974 Nov;37(6):1394–1409. doi: 10.1152/jn.1974.37.6.1394. [DOI] [PubMed] [Google Scholar]
- Henry G. H. Receptive field classes of cells in the striate cortex of the cat. Brain Res. 1977 Sep 9;133(1):1–28. doi: 10.1016/0006-8993(77)90045-2. [DOI] [PubMed] [Google Scholar]
- Hoffman K. P., Stone J. Conduction velocity of afferents to cat visual cortex: a correlation with cortical receptive field properties. Brain Res. 1971 Sep 24;32(2):460–466. doi: 10.1016/0006-8993(71)90340-4. [DOI] [PubMed] [Google Scholar]
- Kelly J. P., Van Essen D. C. Cell structure and function in the visual cortex of the cat. J Physiol. 1974 May;238(3):515–547. doi: 10.1113/jphysiol.1974.sp010541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A. The velocity tuning of single units in cat striate cortex. J Physiol. 1975 Aug;249(3):445–468. doi: 10.1113/jphysiol.1975.sp011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Responses to moving slits by single units in cat striate cortex. Exp Brain Res. 1968;6(4):373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- Singer W., Bedworth N. Inhibitory interaction between X and Y units in the cat lateral geniculate nucleus. Brain Res. 1973 Jan 30;49(2):291–307. doi: 10.1016/0006-8993(73)90424-1. [DOI] [PubMed] [Google Scholar]
- Stone J., Dreher B. Projection of X- and Y-cells of the cat's lateral geniculate nucleus to areas 17 and 18 of visual cortex. J Neurophysiol. 1973 May;36(3):551–567. doi: 10.1152/jn.1973.36.3.551. [DOI] [PubMed] [Google Scholar]


