Abstract
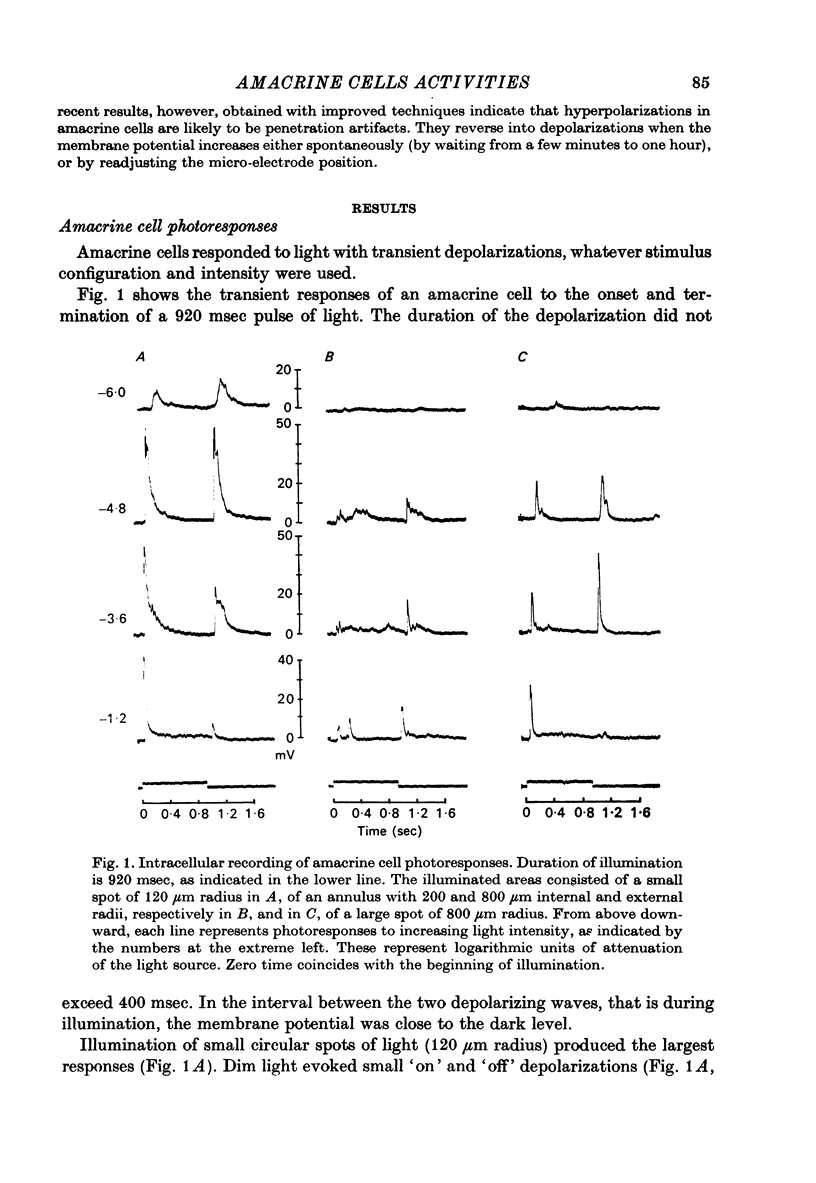
1. Intracellular responses to light were recorded from amacrine cells in the retina of the turtle Pseudemys scripta elegans. 2. The recorded responses were identified on the basis of physiological criteria reported previously (Marchiafava, 1976). Amacrine cells produced transient 'on' and 'off' depolarizing responses irrespective of the retinal area illuminated and of wavelength. 3. The transient depolarizing responses increased by enlarging the illuminated circle up to 120 micrometer in radius. Circles covering larger areas, up to 200 micrometer, produced a relative decrease of the response amplitude. Thus, amacrine cells' receptive fields appear as a central 'excitatory' area of about 120 micrometer radius, surrounded by a 'suppressor' area. 4. Amacrine cells' photoresponses were associated with an increase in membrane conductance. The responses to illumination of central or peripheral areas of the receptive field, however, showed different reversal potentials. The responses to peripheral illumination reversed at about 15 mV above resting potential, while the equilibrium potential of the centre-photoresponses was indicated by extrapolation at about +30 mV. No conductance chance was detectable during steady lights. 5. Repetitive stimulation of the optic nerve invariably reduced amacrine cells' photoresponses, but not those recorded from bipolar cells. It follows then that only ganglion cell photoresponses originating from amacrines' input would be depressed by the nerve stimulation, which thus becomes a reliable test to discriminate whether ganglion cell photoresponses originate from amacrine or bipolar inputs.
Full text
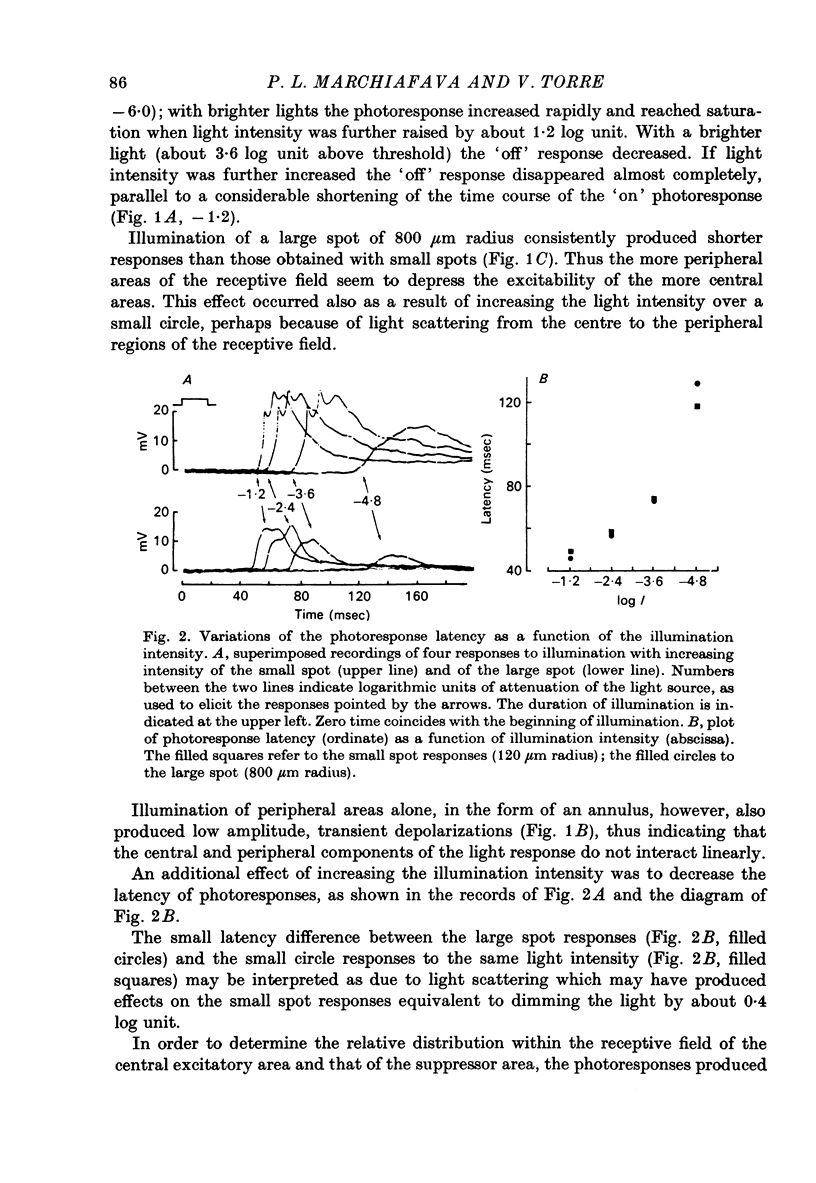
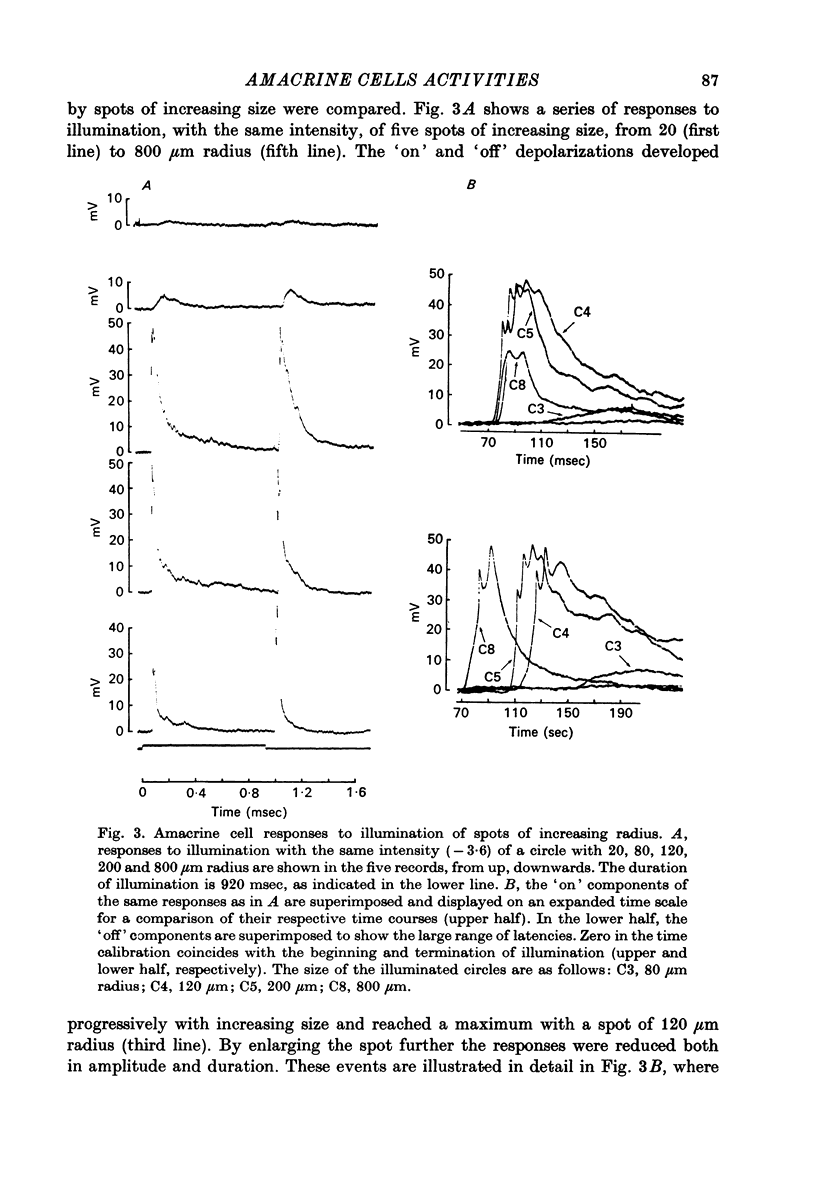
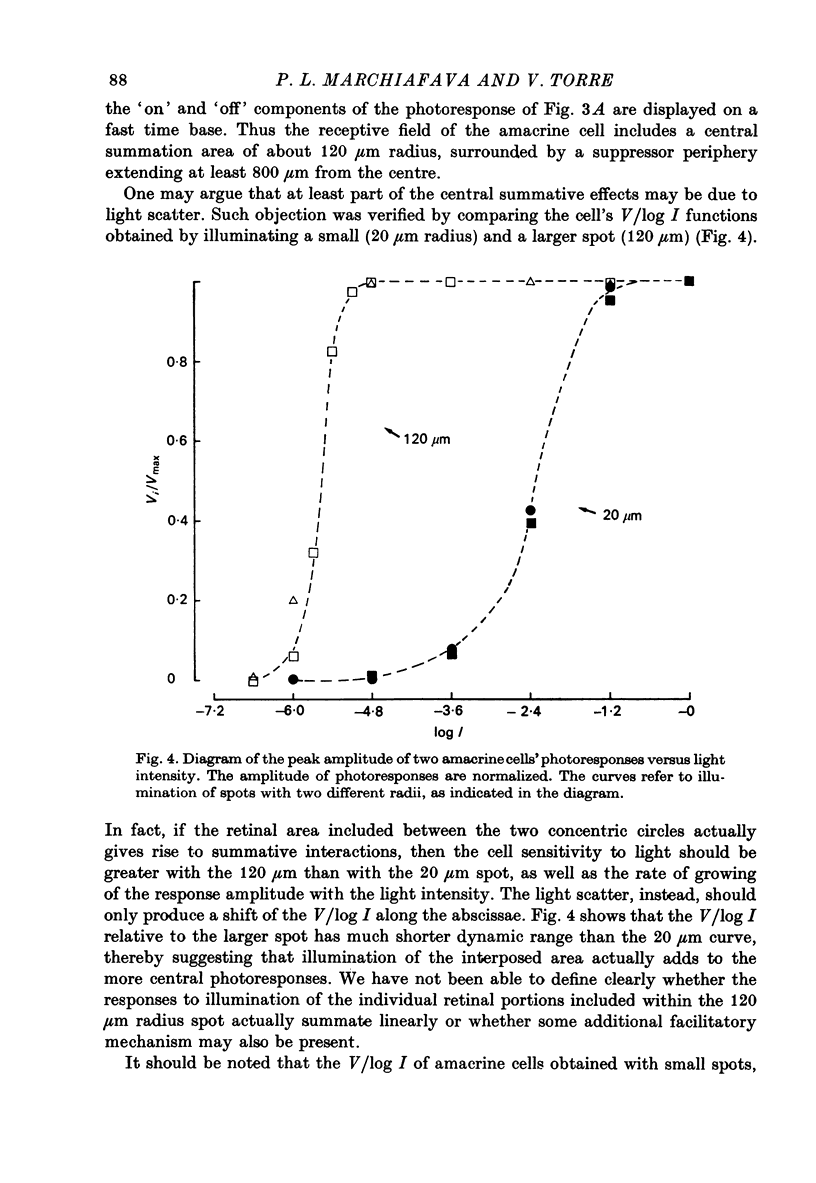
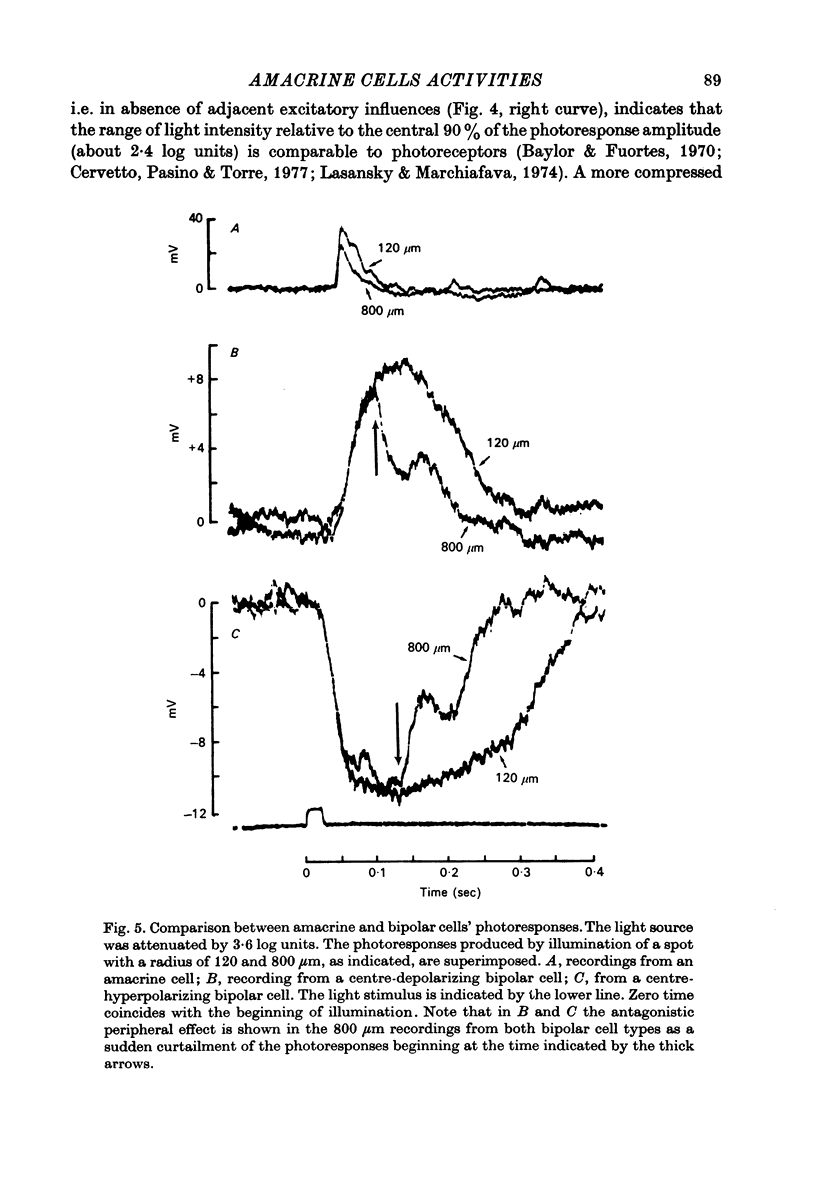
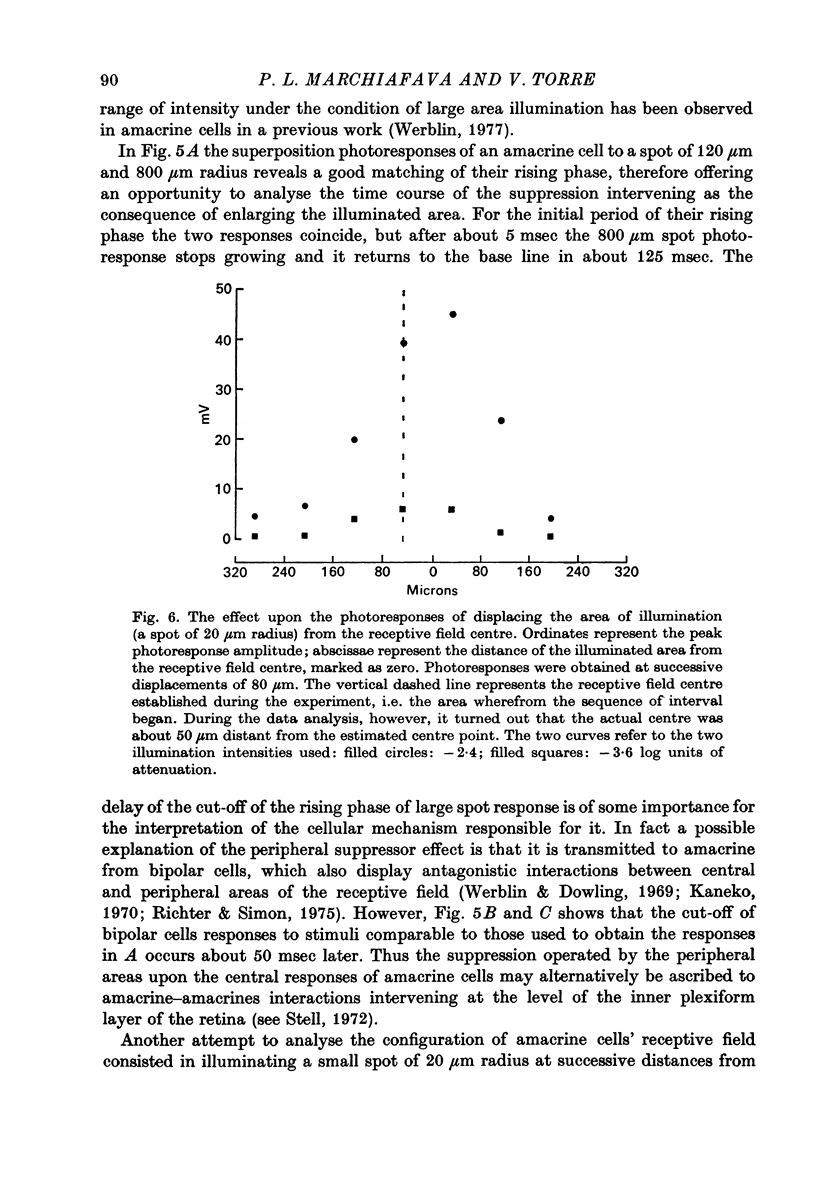
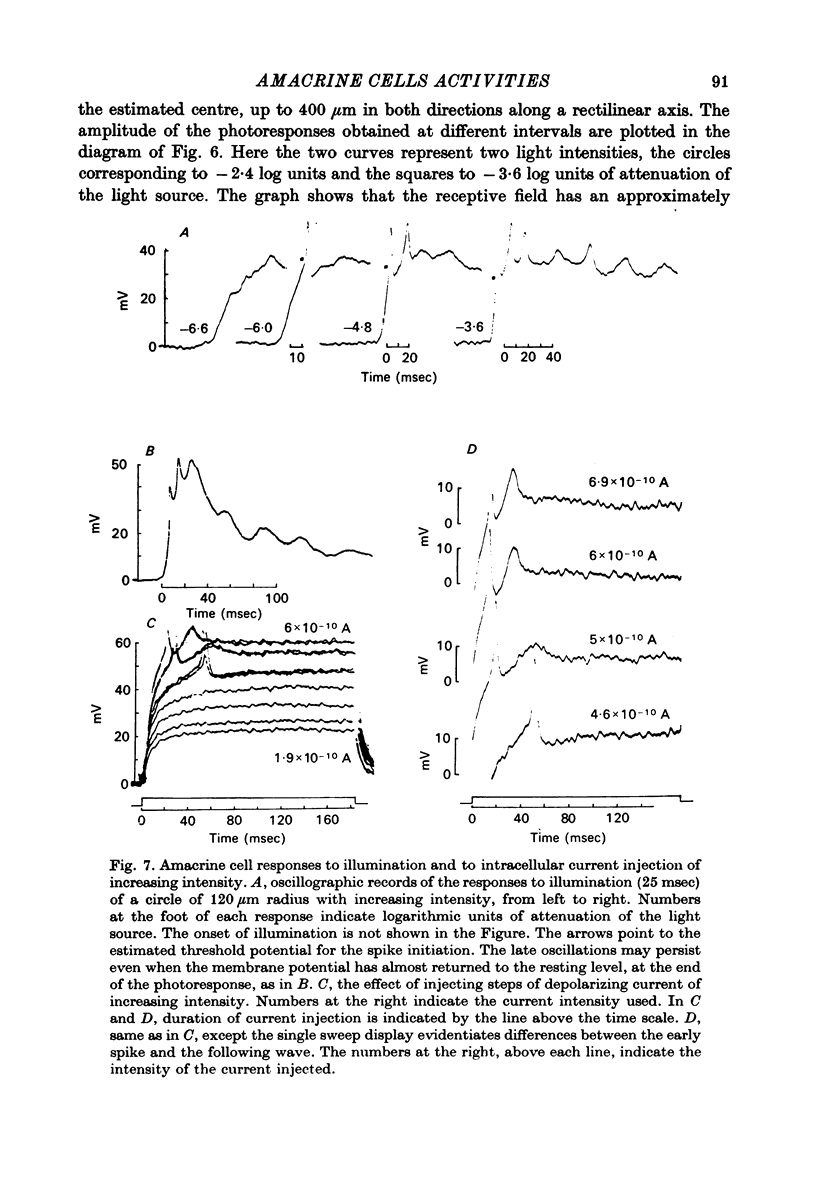
PDF


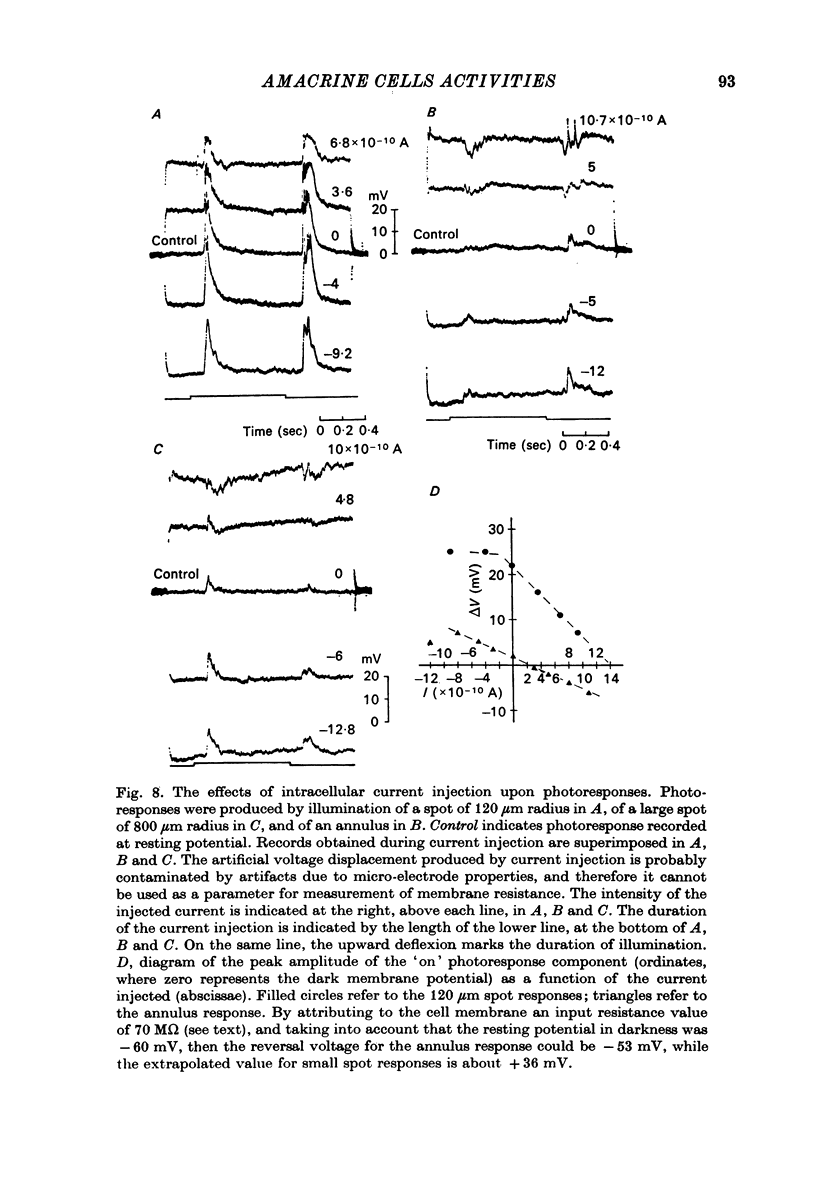

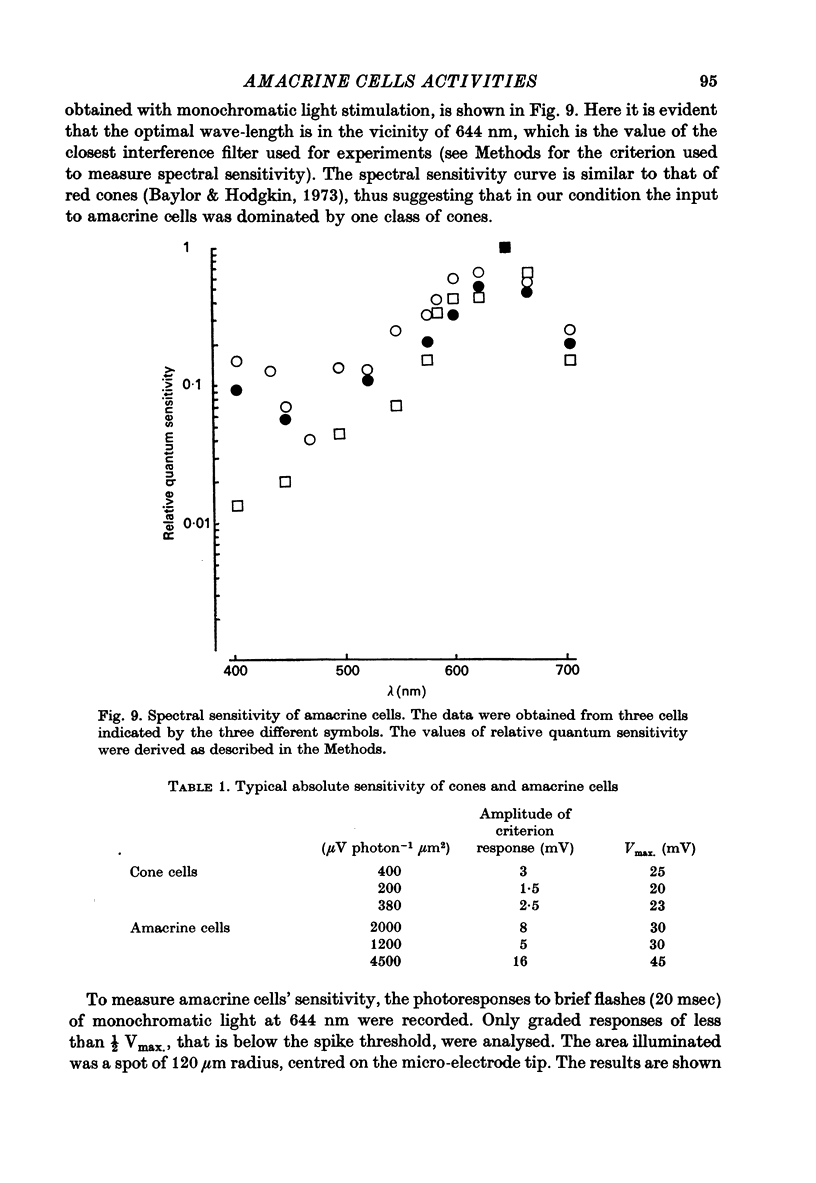
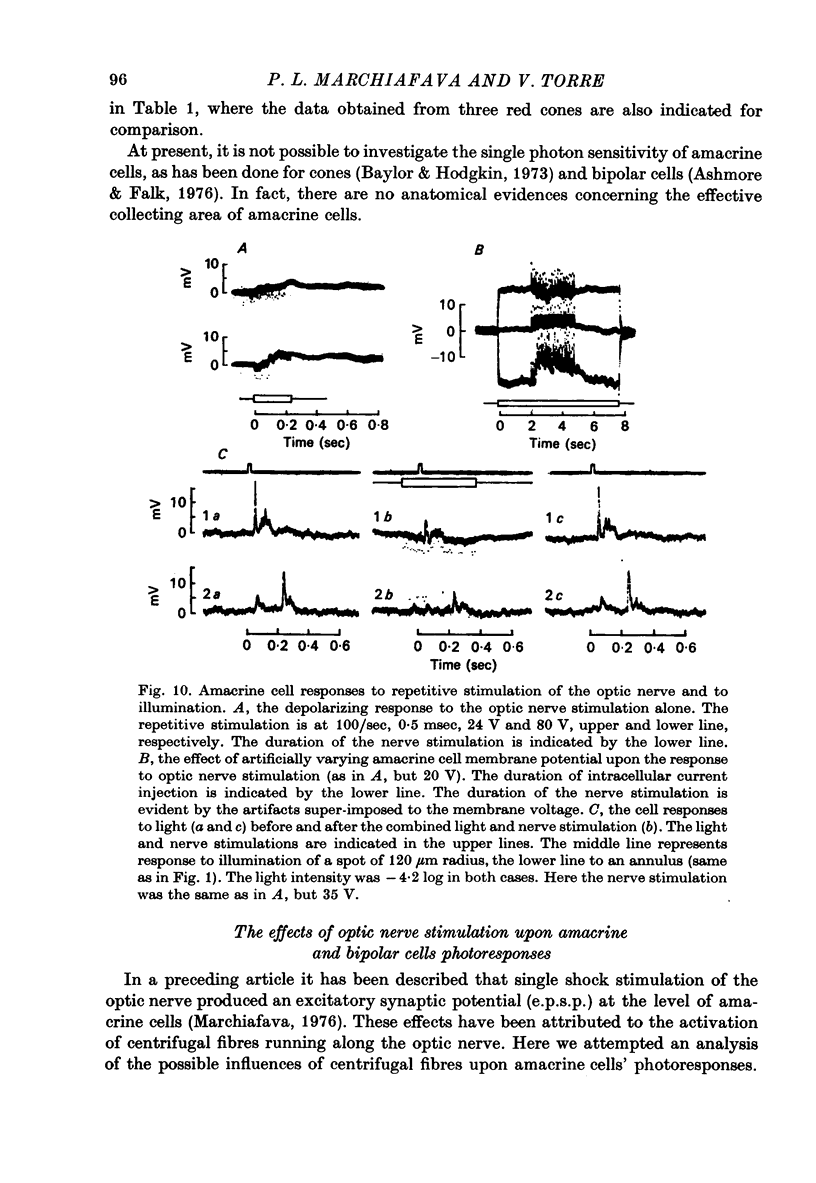
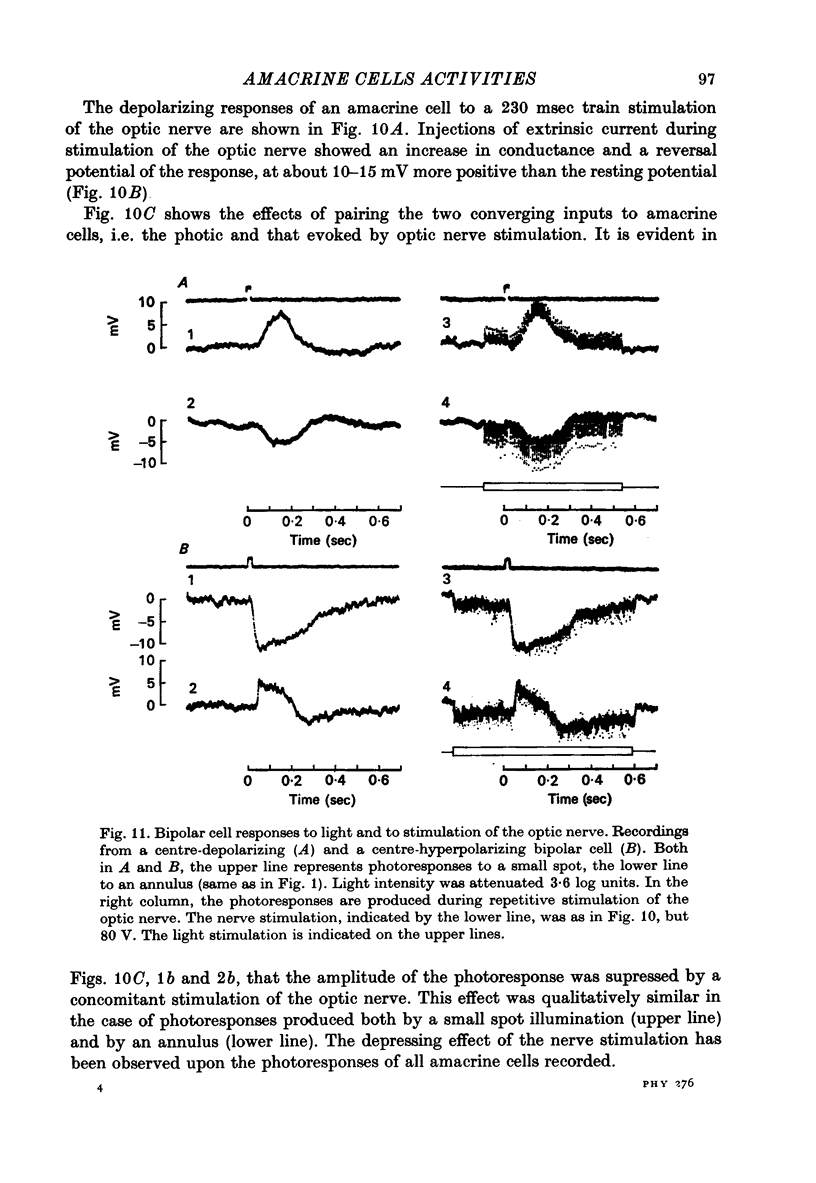

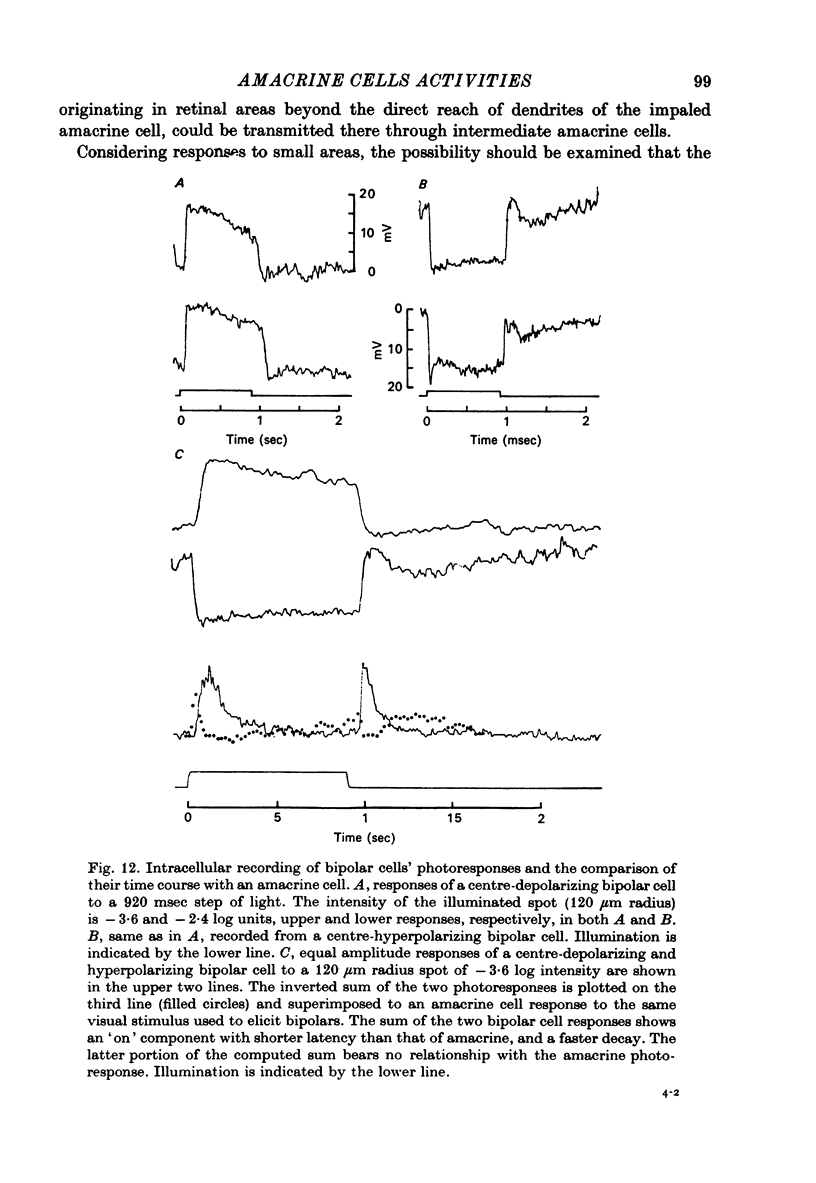


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F., Falk G. Absolute sensitivity of rod bipolar cells in a dark-adapted retina. Nature. 1976 Sep 16;263(5574):248–249. doi: 10.1038/263248a0. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Marchiafava P. L., Pasino E. Influence of efferent retinal fibres on responsiveness of ganglion cells to light. Nature. 1976 Mar 4;260(5546):56–57. doi: 10.1038/260056a0. [DOI] [PubMed] [Google Scholar]
- Cervetto L., Pasino E., Torre V. Electrical responses of rods in the retina of Bufo marinus. J Physiol. 1977 May;267(1):17–51. doi: 10.1113/jphysiol.1977.sp011799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. Y., Naka K. The amacrine cell. Vision Res. 1976;16(10):1119–1129. doi: 10.1016/0042-6989(76)90252-2. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L. The local electric changes associated with repetitive action in a non-medullated axon. J Physiol. 1948 Mar 15;107(2):165–181. doi: 10.1113/jphysiol.1948.sp004260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Hashimoto H. Electrophysiological study of single neurons in the inner nuclear layer of the carp retina. Vision Res. 1969 Jan;9(1):37–55. doi: 10.1016/0042-6989(69)90030-3. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A. Receptive field organization of bipolar and amacrine cells in the goldfish retina. J Physiol. 1973 Nov;235(1):133–153. doi: 10.1113/jphysiol.1973.sp010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of prolonged depolarization on synaptic transfer in the stellate ganglion of the squid. J Physiol. 1971 Jul;216(2):503–512. doi: 10.1113/jphysiol.1971.sp009537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A., Marchiafava P. L. Light-induced resistance changes in retinal rods and cones of the tiger salamander. J Physiol. 1974 Jan;236(1):171–191. doi: 10.1113/jphysiol.1974.sp010429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiafava P. L. Centrifugal actions on amacrine and ganglion cells in the retina of the turtle. J Physiol. 1976 Feb;255(1):137–155. doi: 10.1113/jphysiol.1976.sp011273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiafava P. L., Torre V. Self-facilitation of ganglion cells in the retina of the turtle. J Physiol. 1977 Jun;268(2):335–351. doi: 10.1113/jphysiol.1977.sp011860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. F., Dacheux R. F. Synaptic organization and ionic basis of on and off channels in mudpuppy retina. I. Intracellular analysis of chloride-sensitive electrogenic properties of receptors, horizontal cells, bipolar cells, and amacrine cells. J Gen Physiol. 1976 Jun;67(6):639–659. doi: 10.1085/jgp.67.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Shimoda Y. Identification of amacrine and ganglion cells in the carp retina. J Physiol. 1977 Jan;264(3):801–818. doi: 10.1113/jphysiol.1977.sp011695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A., Simon E. J. Properties of centre-hyperpolarizing, red-sensitive bipolar cells in the turtle retina. J Physiol. 1975 Jun;248(2):317–334. doi: 10.1113/jphysiol.1975.sp010976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda J., Hashimoto H., Otsu K. Bipolar-amacrine transmission in the carp retina. Vision Res. 1973 Feb;13(2):295–307. doi: 10.1016/0042-6989(73)90108-9. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Copenhagen D. R. Control of retinal sensitivity. 3. Lateral interactions at the inner plexiform layer. J Gen Physiol. 1974 Jan;63(1):88–110. doi: 10.1085/jgp.63.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Regenerative amacrine cell depolarization and formation of on-off ganglion cell response. J Physiol. 1977 Jan;264(3):767–785. doi: 10.1113/jphysiol.1977.sp011693. [DOI] [PMC free article] [PubMed] [Google Scholar]


