Abstract
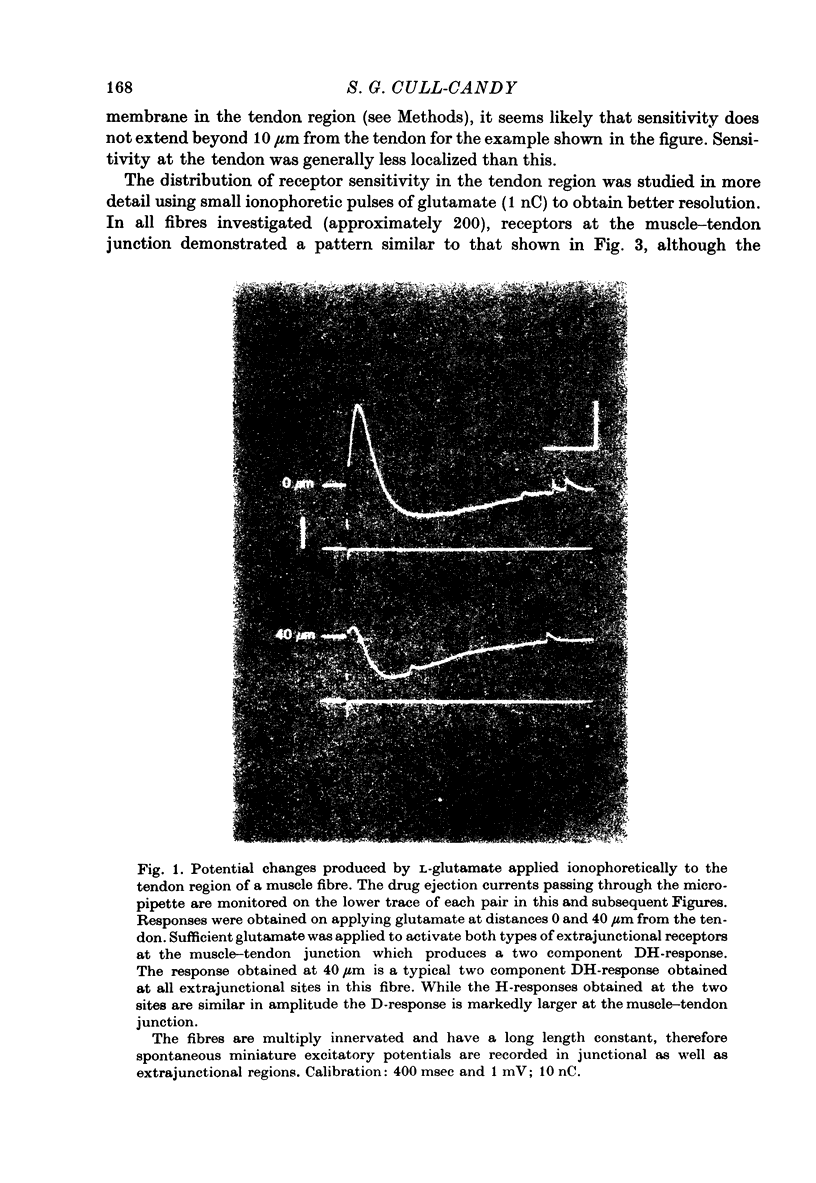
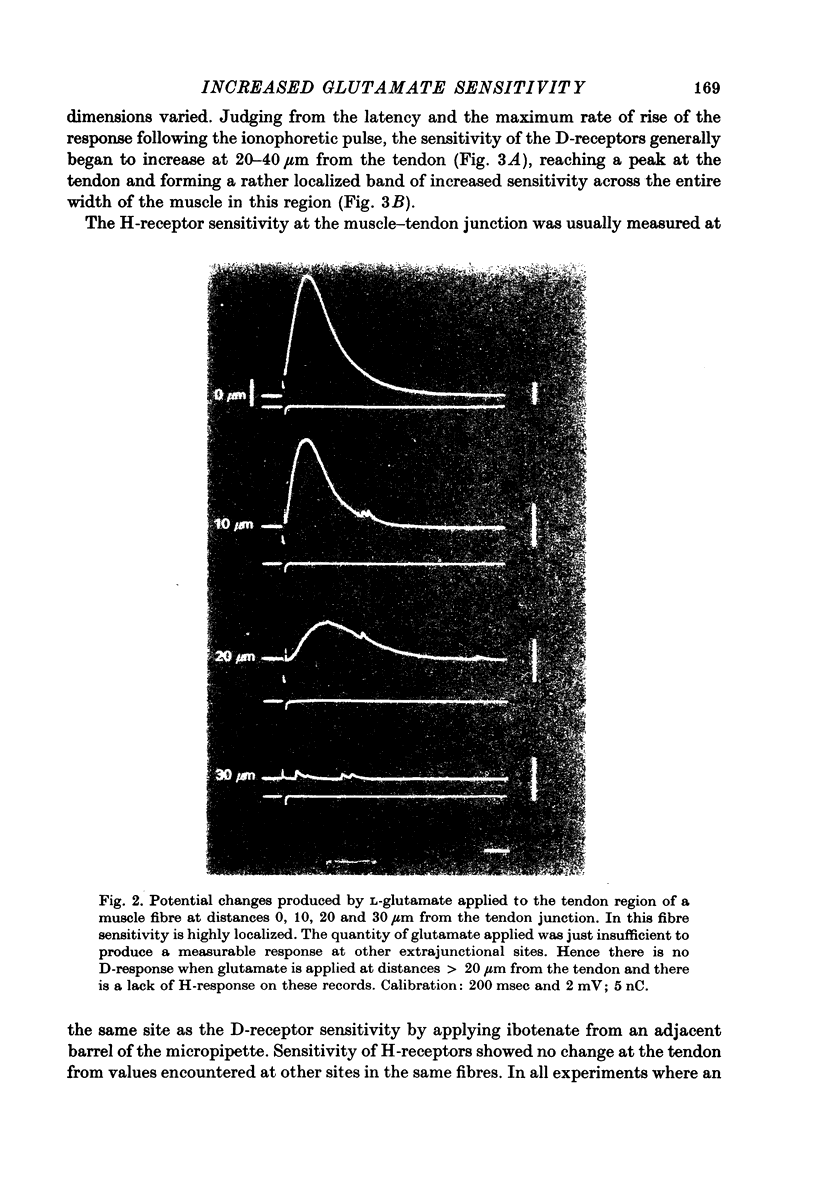
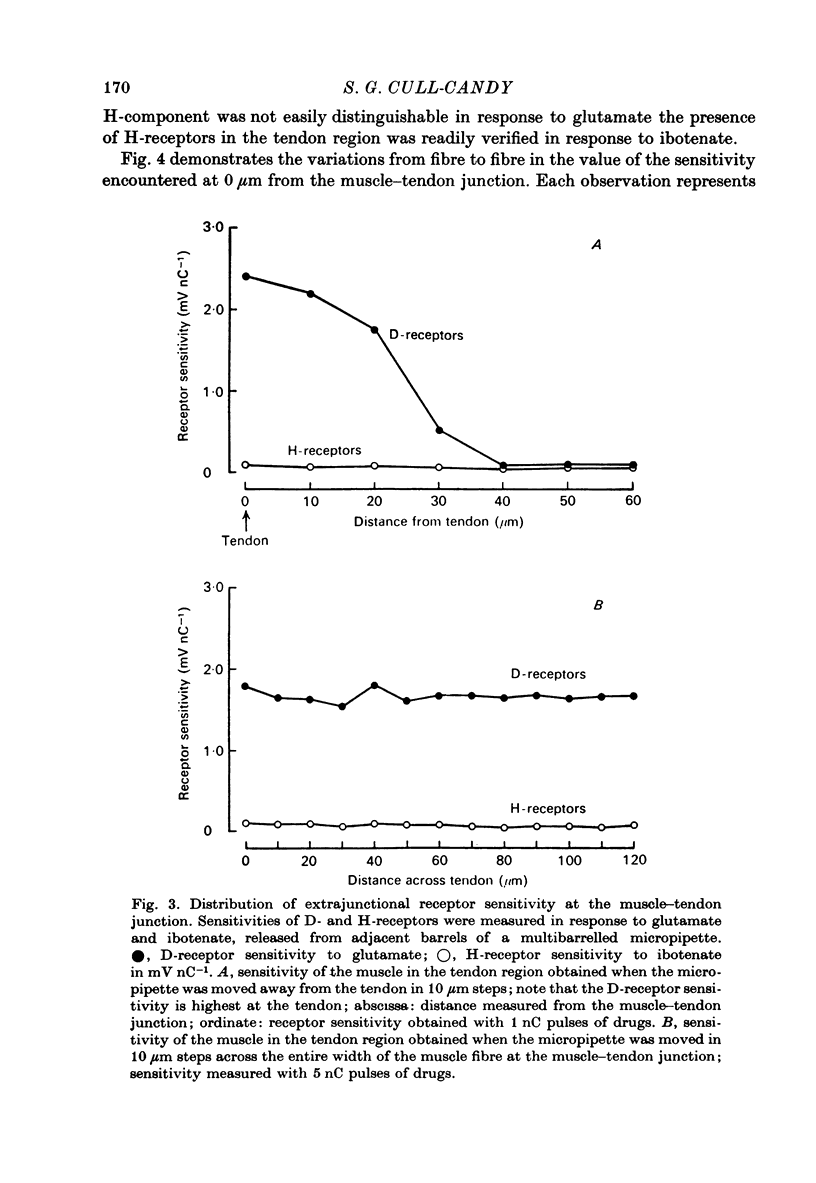
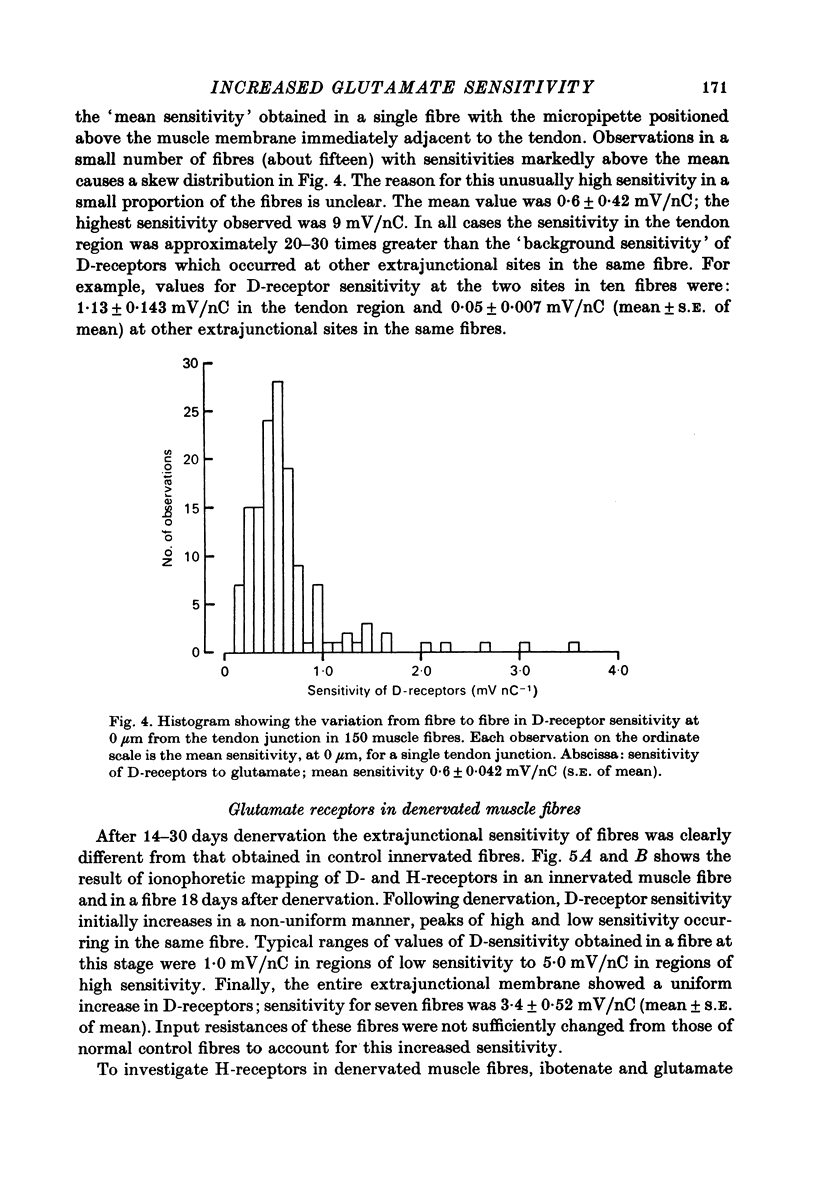
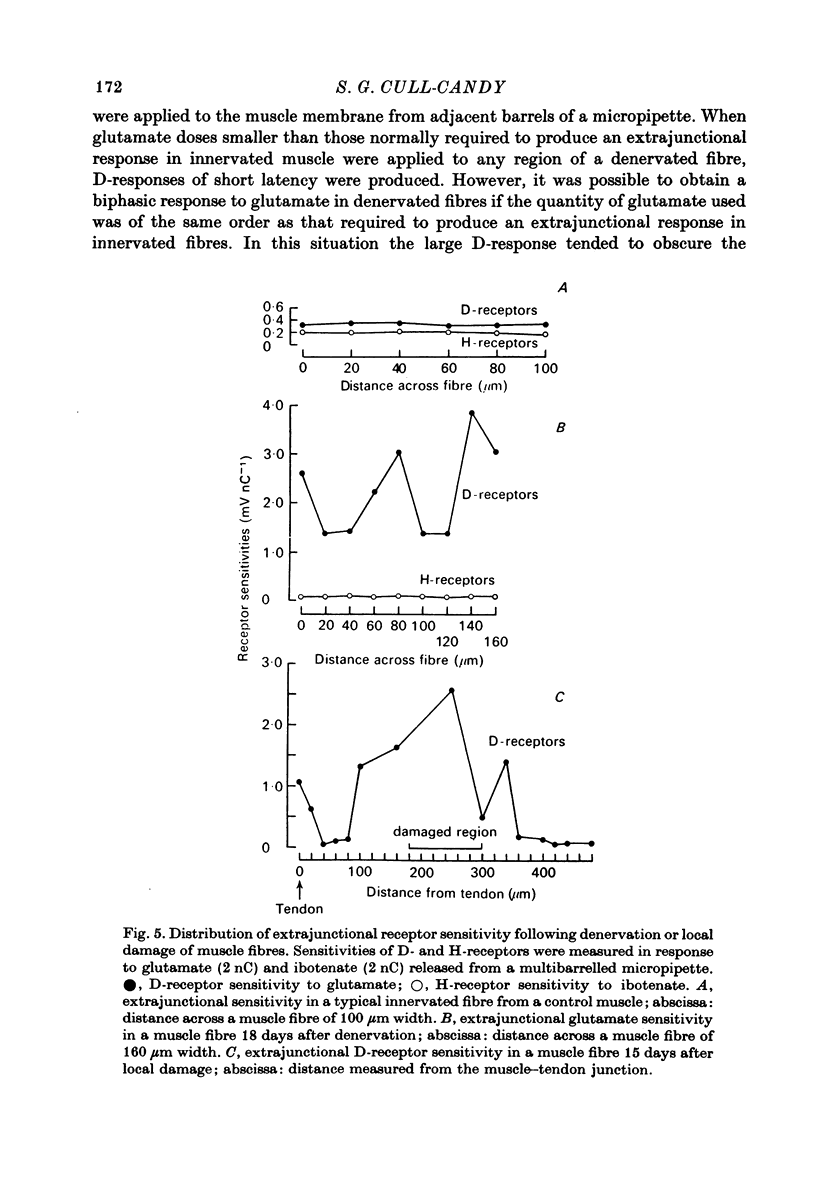
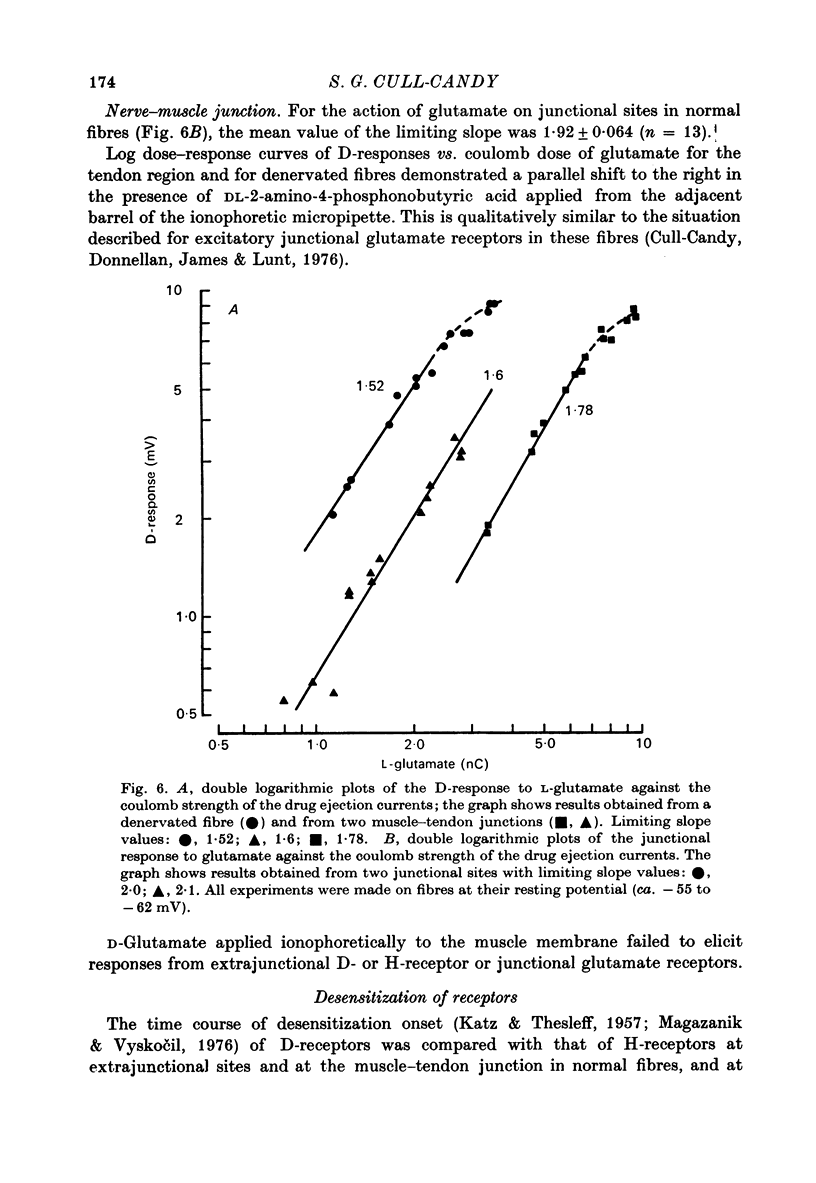
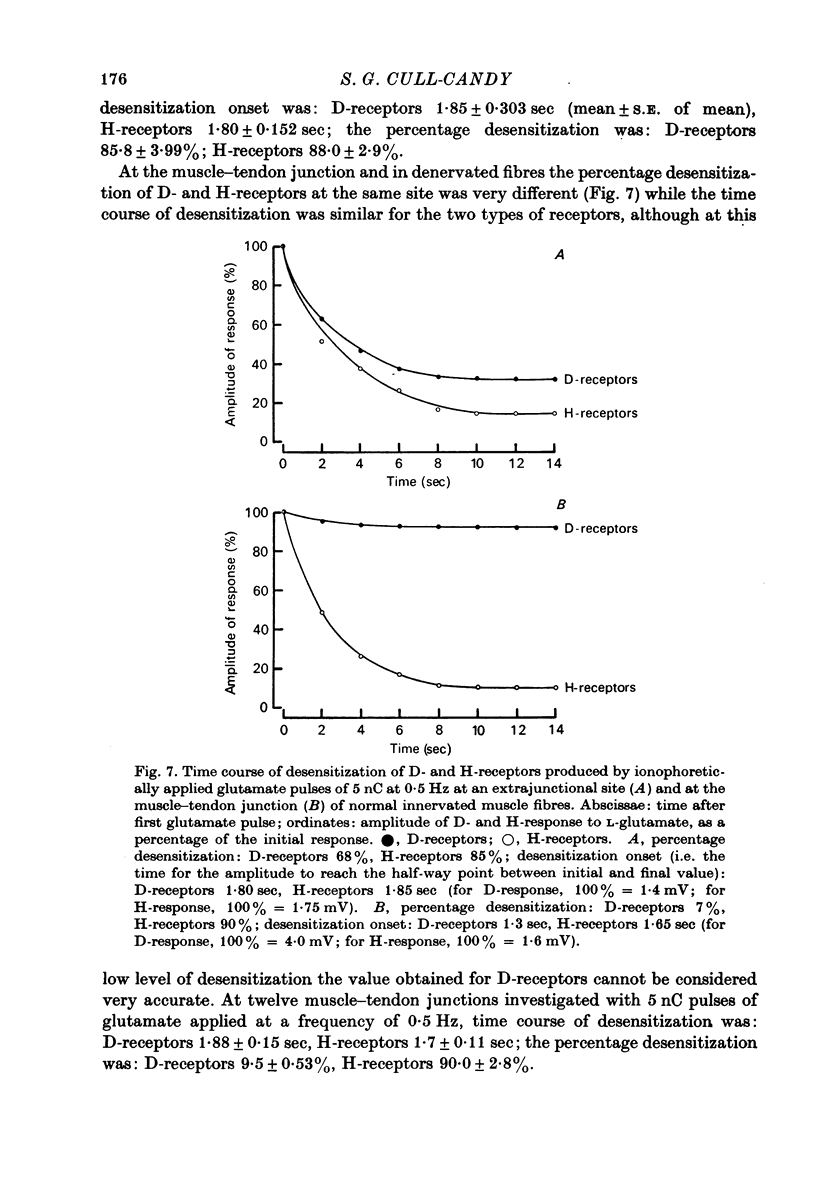
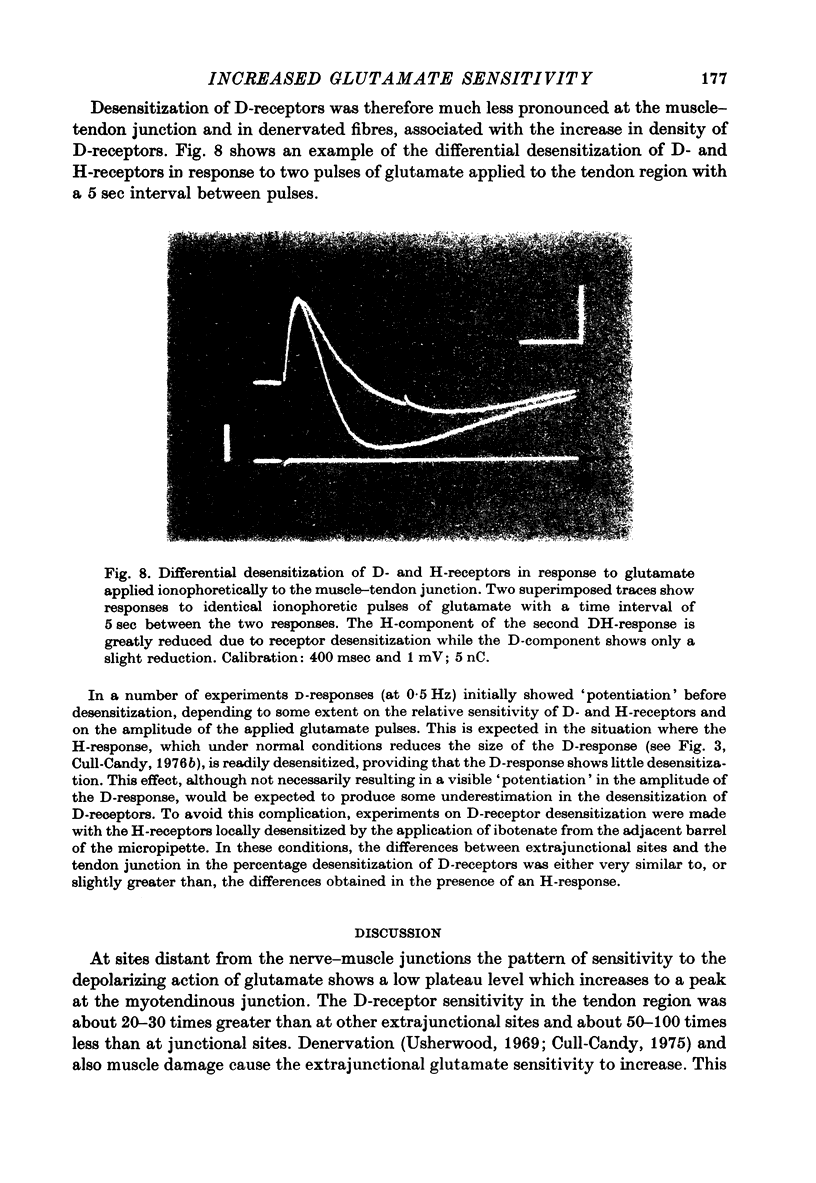
1. Factors influencing the glutamate sensitivity of extrajunctional regions of innervated and denervated locust muscle fibres have been investigated. Properties of the two types of extrajunctional glutamate receptors, D- and H-receptors, have been studied in regions of high and low sensitivity. 2. The low level of extrajunctional sensitivity which is normally present in innervated fibres was 20-30 times higher at the muscle-tendon junction than at other sites; increased sensitivity extended about 20-40 micron from the tendon. After denervation or localized damage the entire extrajunctional sensitivity was increased approximately 100 times above control levels. 3. Applying L-glutamate (which activates D- and H-receptors) and DL-ibotenate (which activates H-receptors) from multibarrelled micropipettes showed that increased extrajunctional sensitivity resulted from an increase in D-receptors while H-receptors were apparently unchanged. 4. Coulomb dose vs. response relationships for the action of glutamate on D-receptors were similar when obtained at the muscle-tendon junction and nerve-muscle junction of innervated fibres or at extrajunctional regions in denervated fibres. 5. Time course of onset and percentage desensitization of D- and H-receptors in innervated fibres were similar. The percentage desensitization of D-receptors in extrajunctional regions of high sensitivity was greatly reduced. 6. It is suggested that D- and H-receptors are independent and that the trigger for increased receptor sensitivity acts specifically on D-receptors. In all respects so far studied, the D-receptors resemble extrajunctional ACh-receptors in vertebrate muscle.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Cull-Candy S. G., Miledi R. Glutamate and quisqualate noise in voltage-clamped locust muscle fibres. Nature. 1976 May 13;261(5556):151–153. doi: 10.1038/261151a0. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., WATKINS J. C. The excitation and depression of spinal neurones by structurally related amino acids. J Neurochem. 1960 Sep;6:117–141. doi: 10.1111/j.1471-4159.1960.tb13458.x. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G. Effect of denervation and local damage on extrajunctional L-glutamate receptors in locust muscle. Nature. 1975 Dec 11;258(5535):530–531. doi: 10.1038/258530a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G. Two types of extrajunctional L-glutamate receptors in locust muscle fibres. J Physiol. 1976 Feb;255(2):449–464. doi: 10.1113/jphysiol.1976.sp011289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Walther C., Peper K. Junctional and extrajunctional acetylcholine receptors in normal and denervated frog muscle fibres. Noise analysis experiments with different agonists. Pflugers Arch. 1976 Oct 15;366(1):1–9. doi: 10.1007/BF02486555. [DOI] [PubMed] [Google Scholar]
- Dudel J. Dose-response curve of glutamate applied by superfusion to crayfish muscle synapses. Pflugers Arch. 1977 Mar 11;368(1-2):49–54. doi: 10.1007/BF01063454. [DOI] [PubMed] [Google Scholar]
- Dudel J. Potentiation and desensitization after glutamate induced postsynaptic currents at the crayfish neuromuscular junction. Pflugers Arch. 1975;356(4):317–327. doi: 10.1007/BF00580005. [DOI] [PubMed] [Google Scholar]
- Faeder I. R., Salpeter M. M. Glutamate uptake by a stimulated insect nerve muscle preparation. J Cell Biol. 1970 Aug;46(2):300–307. doi: 10.1083/jcb.46.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E. The sensitivity to glutamate of denervated muscles of the crayfish. J Physiol. 1974 Oct;242(2):371–382. doi: 10.1113/jphysiol.1974.sp010712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann E. Neurotrophic relations. Annu Rev Physiol. 1976;38:177–216. doi: 10.1146/annurev.ph.38.030176.001141. [DOI] [PubMed] [Google Scholar]
- HOYLE G. The anatomy and innervation of locust skeletal muscle. Proc R Soc Lond B Biol Sci. 1955 Jan 27;143(911):281–292. doi: 10.1098/rspb.1955.0011. [DOI] [PubMed] [Google Scholar]
- James R. W., Lunt G. G., Donnellan J. F. Isolation of a glutaminergic receptor fraction from locust muscle. Biochem Soc Trans. 1977;5(1):170–172. doi: 10.1042/bst0050170. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Curtis D. R., Davies J., McCulloch R. M. Spinal interneurone excitation by conformationally restricted analogues of L-glutamic acid. Nature. 1974 Apr 26;248(5451):804–805. doi: 10.1038/248804a0. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. FURTHER OBSERVATIONS ON THE DISTRIBUTION OF ACTYLCHOLINE-REACTIVE SITES IN SKELETAL MUSCLE. J Physiol. 1964 Mar;170:379–388. doi: 10.1113/jphysiol.1964.sp007338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE DEVELOPMENT OF ACETYLCHOLINE SENSITIVITY IN NERVE-FREE SEGMENTS OF SKELETAL MUSCLE. J Physiol. 1964 Mar;170:389–396. doi: 10.1113/jphysiol.1964.sp007339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Dennis M. J., Harris A. J. The development of chemosensitivity in extrasynaptic areas of the neuronal surface after denervation of parasympathetic ganglion cells in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):555–563. doi: 10.1098/rspb.1971.0047. [DOI] [PubMed] [Google Scholar]
- Langer S. Z., Trendelenburg U. The effect of a saturable uptake mechanism on the slopes of dose-response curves for sympathomimetic amines and on the shifts of dose-response curves produced by a competitive antagonist. J Pharmacol Exp Ther. 1969 May;167(1):117–142. [PubMed] [Google Scholar]
- Lawn I. D. Swimming in the sea anemone Stomphia coccinea triggered by a slow conduction system. Nature. 1976 Aug 19;262(5570):708–709. doi: 10.1038/262708a0. [DOI] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocit F. The effect of temperature on desensitization kinetics at the post-synaptic membrane of the frog muscle fibre. J Physiol. 1975 Jul;249(2):285–300. doi: 10.1113/jphysiol.1975.sp011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Stefani E., Zelená J. Neural control of acetylcholine-sensitivity in rat muscle fibers. Nature. 1968 Nov 2;220(5166):497–498. doi: 10.1038/220497a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Noise analysis of drug induced voltage clamp currents in denervated frog muscle fibres. J Physiol. 1976 Jul;258(3):705–729. doi: 10.1113/jphysiol.1976.sp011442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper S. The acetylcholine sensitivity of the surface membrane of multiply-innervated parasympathetic ganglion cells in the mudpuppy before and after partial denervation. J Physiol. 1976 Jan;254(2):455–473. doi: 10.1113/jphysiol.1976.sp011240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M., Zieglgänsberger W., Herz A. Supersensitivity of cortical neurones of the rat to acetylcholine and L-glutamate following chronic morphine treatment. Naunyn Schmiedebergs Arch Pharmacol. 1976;293(1):101–103. doi: 10.1007/BF00498877. [DOI] [PubMed] [Google Scholar]
- Shinozaki H., Konishi S. Actions of several anthelmintics and insecticides on rat cortical neurones. Brain Res. 1970 Dec 1;24(2):368–371. doi: 10.1016/0006-8993(70)90122-8. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. THE EFFECT ON CRAYFISH MUSCLE OF IONTOPHORETICALLY APPLIED GLUTAMATE. J Physiol. 1964 Mar;170:296–317. doi: 10.1113/jphysiol.1964.sp007332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. A study of the action of picrotoxin on the inhibitory neuromuscular junction of the crayfish. J Physiol. 1969 Nov;205(2):377–391. doi: 10.1113/jphysiol.1969.sp008972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usherwood P. N. Glutamate sensitivity of denervated insect muscle fibres. Nature. 1969 Jul 26;223(5204):411–413. doi: 10.1038/223411a0. [DOI] [PubMed] [Google Scholar]
- Walker R. J., Woodruff G. N., Kerkut G. A. The effect of ibotenic acid and muscimol on single neurons of the snail, Helix aspersa. Comp Gen Pharmacol. 1971 Jun;2(6):168–174. doi: 10.1016/0010-4035(71)90007-3. [DOI] [PubMed] [Google Scholar]


