Abstract
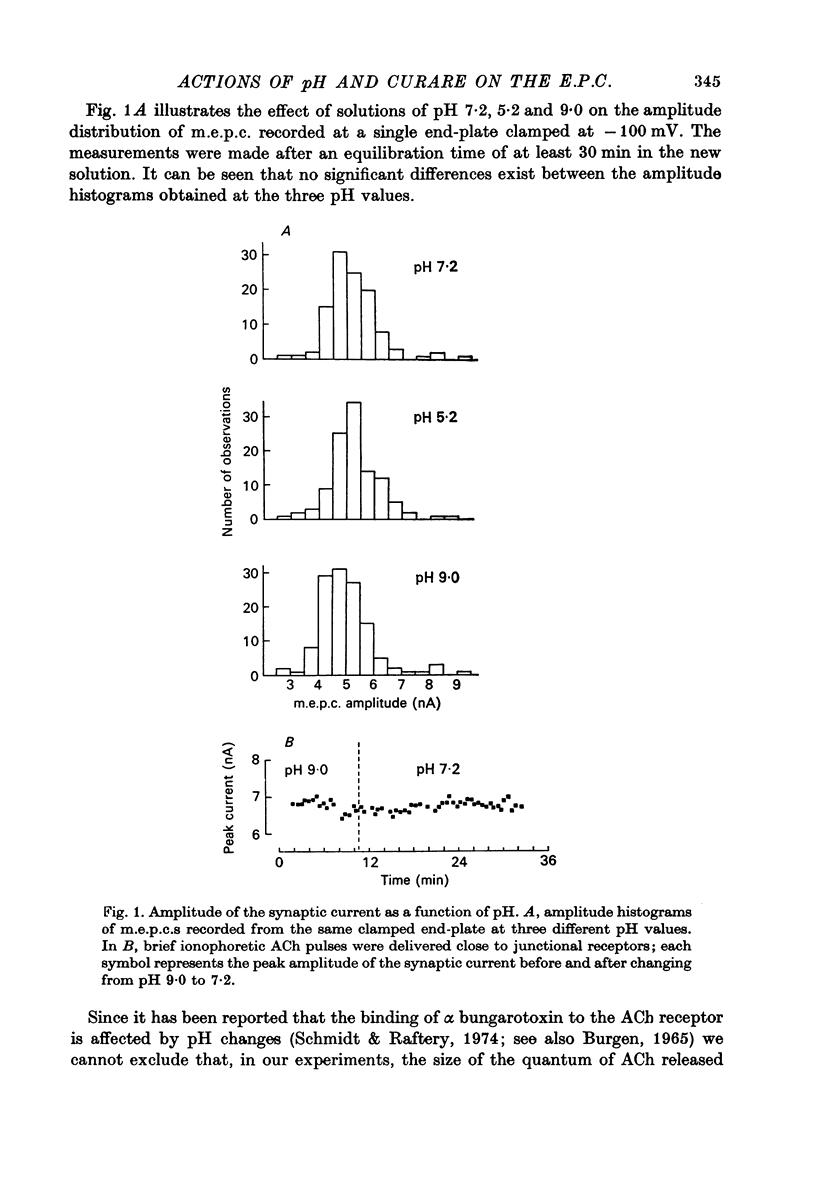
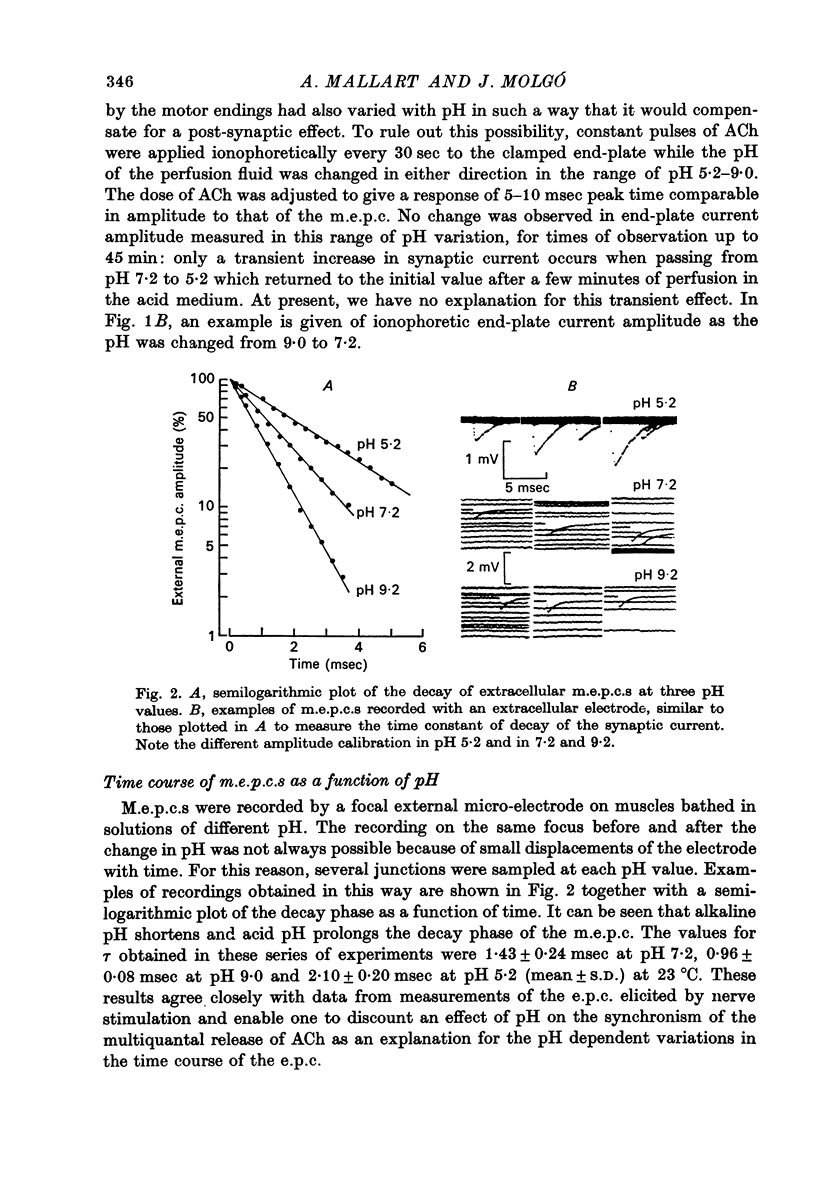
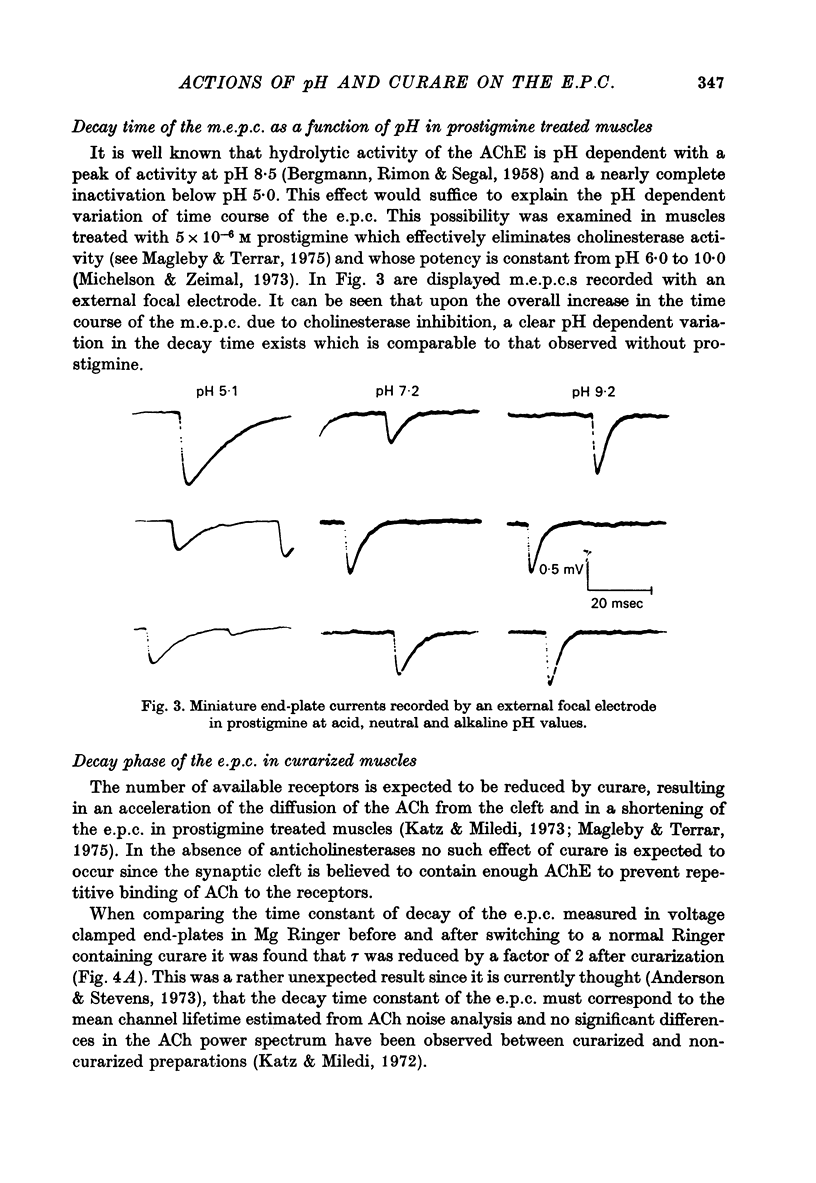
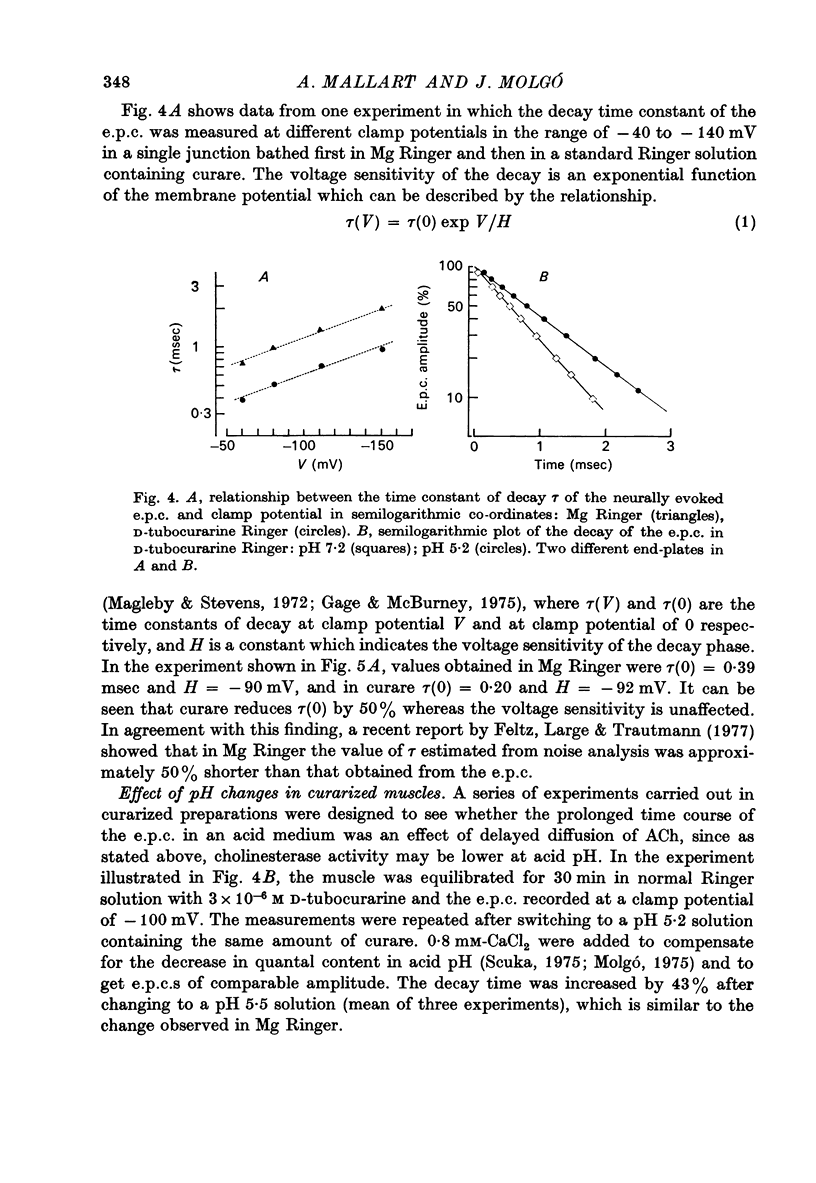
1. The effect of pH changes on synaptic currents has been analysed by external recording of the miniature end-plate currents (m.e.p.c.s) or by recording in voltage-clamped end-plates the current elicited by nerve stimulation (e.p.c.). 2. Changes in pH do not appreciably effect the peak amplitude of the current produced by a single quantum or by short ionophoretic pulses of acetylcholine. 3. The time constant of decay of the m.e.p.c.s is prolonged by about 50% in acid pH and shortened by about the same amount in alkaline pH. This effect is independent of the cholinesterase activity of the end-plate. 4. In curarized preparations the decay of the e.p.c. is shorter than in Mg-blocked end-plate even in the absence of cholinesterase blocking agents. 5. The action of pH on the decays can be explained by a titration of the surface charges of the membrane which effects the voltage dependent reaction that controls the rate of closing of the synaptic channels.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Relaxation experiments using bath-applied suberyldicholine. J Physiol. 1977 Jun;268(2):271–289. doi: 10.1113/jphysiol.1977.sp011857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGMANN F., RIMON S., SEGAL R. Effect of pH on the activity of eel esterase towards different substrates. Biochem J. 1958 Mar;68(3):493–499. doi: 10.1042/bj0680493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Haim D., Dreyer F., Peper K. Acetylcholine receptor: modification of synaptic gating mechanism after treatment with a disulfide bond reducing agent. Pflugers Arch. 1975 Mar 22;355(1):19–26. doi: 10.1007/BF00584796. [DOI] [PubMed] [Google Scholar]
- Cohen I., Van Der Kloot W. The effects of pH changes on the frequency of miniature end-plate potentials at the frog neuromuscular junction. J Physiol. 1976 Nov;262(2):401–414. doi: 10.1113/jphysiol.1976.sp011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Large W. A., Trautmann A. Analysis of atropine action at the frog neutromuscular junction. J Physiol. 1977 Jul;269(1):109–130. doi: 10.1113/jphysiol.1977.sp011895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965 Dec;181(3):656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The nature of the prolonged endplate depolarization in anti-esterase treated muscle. Proc R Soc Lond B Biol Sci. 1975 Dec 31;192(1106):27–38. doi: 10.1098/rspb.1975.0149. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau E. M., Ben-Haim D. Acetylcholine noise: analysis after chemical modification of receptor. Science. 1974 Sep 13;185(4155):944–946. doi: 10.1126/science.185.4155.944. [DOI] [PubMed] [Google Scholar]
- Landau E. M., Nachshen D. A. The interaction of pH and divalent cations at the neuromuscular junction. J Physiol. 1975 Oct;251(3):775–790. doi: 10.1113/jphysiol.1975.sp011121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Terrar D. A. Factors affecting the time course of decay of end-plate currents: a possible cooperative action of acetylcholine on receptors at the frog neuromuscular junction. J Physiol. 1975 Jan;244(2):467–495. doi: 10.1113/jphysiol.1975.sp010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Dreyer F., Peper K. Current-voltage relation and reversal potential at junctional and extrajunctional ACh-receptors of the frog neuromuscular junction. Pflugers Arch. 1976 Mar 11;362(1):43–47. doi: 10.1007/BF00588679. [DOI] [PubMed] [Google Scholar]
- Marnay A., Nachmansohn D. Choline esterase in voluntary muscle. J Physiol. 1938 Feb 16;92(1):37–47. doi: 10.1113/jphysiol.1938.sp003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J., Raftery M. A. The cation sensitivity of the acetylcholine receptor from Torpedo californica. J Neurochem. 1974 Oct;23(4):617–623. doi: 10.1111/j.1471-4159.1974.tb04383.x. [DOI] [PubMed] [Google Scholar]
- Terrar D. A. The effect of dithiothreitol on the time course of end-plate currents in the presence of neostigmine [proceedings]. J Physiol. 1976 Dec;263(1):119P–120P. [PubMed] [Google Scholar]
- Weiss L. Cell contact phenomena. In Vitro. 1970;5:48–78. doi: 10.1007/BF02618374. [DOI] [PubMed] [Google Scholar]


