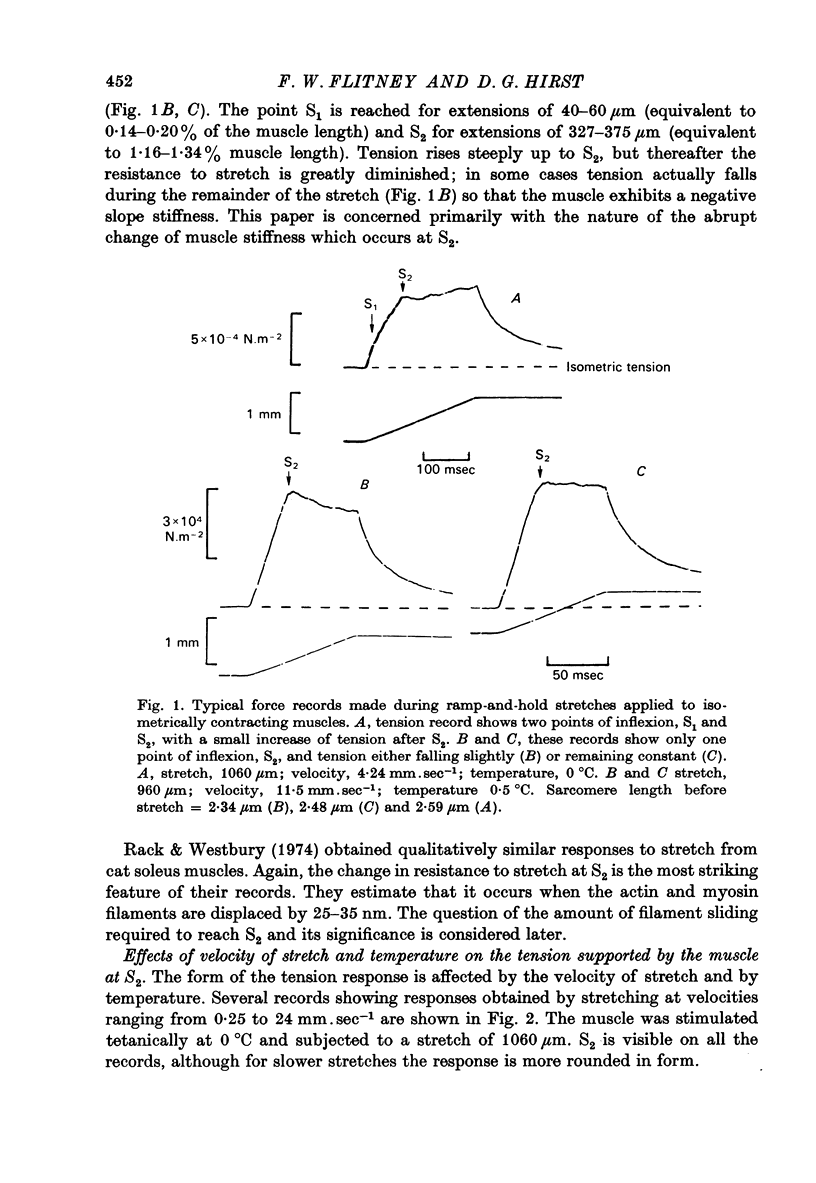
Abstract
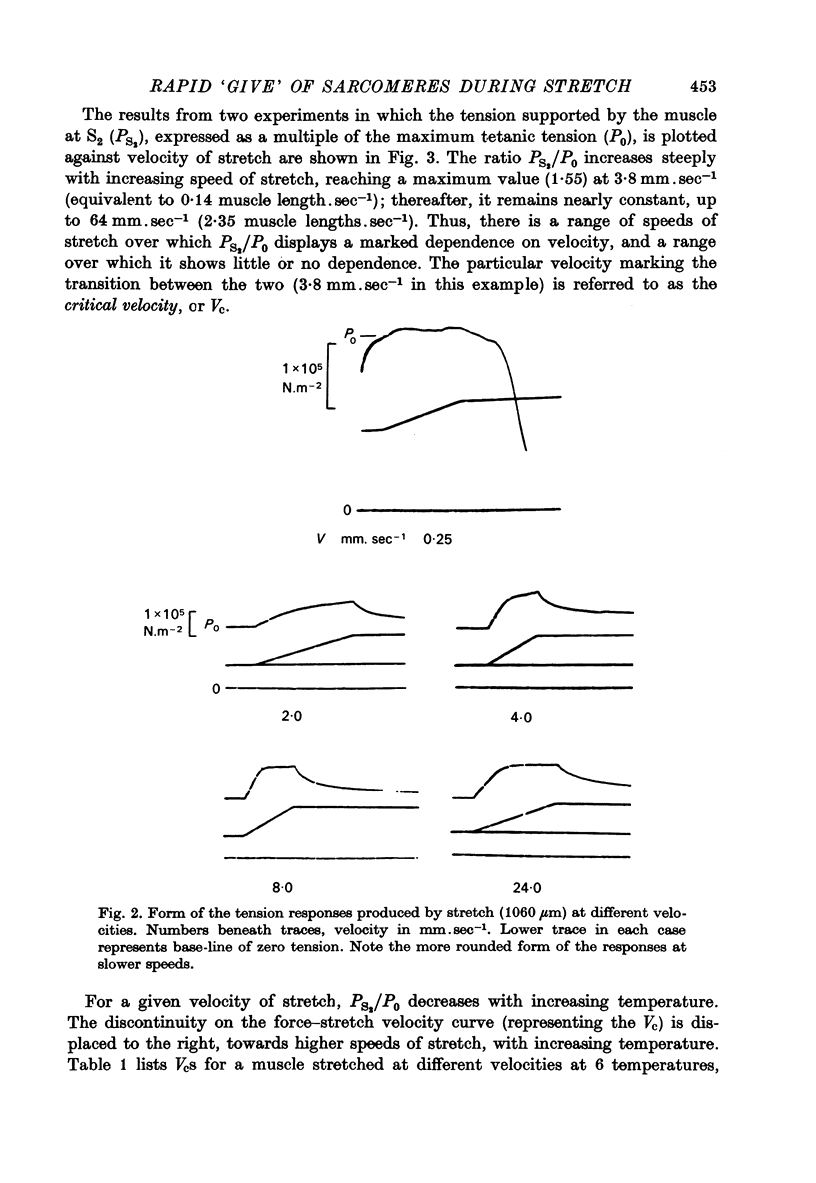
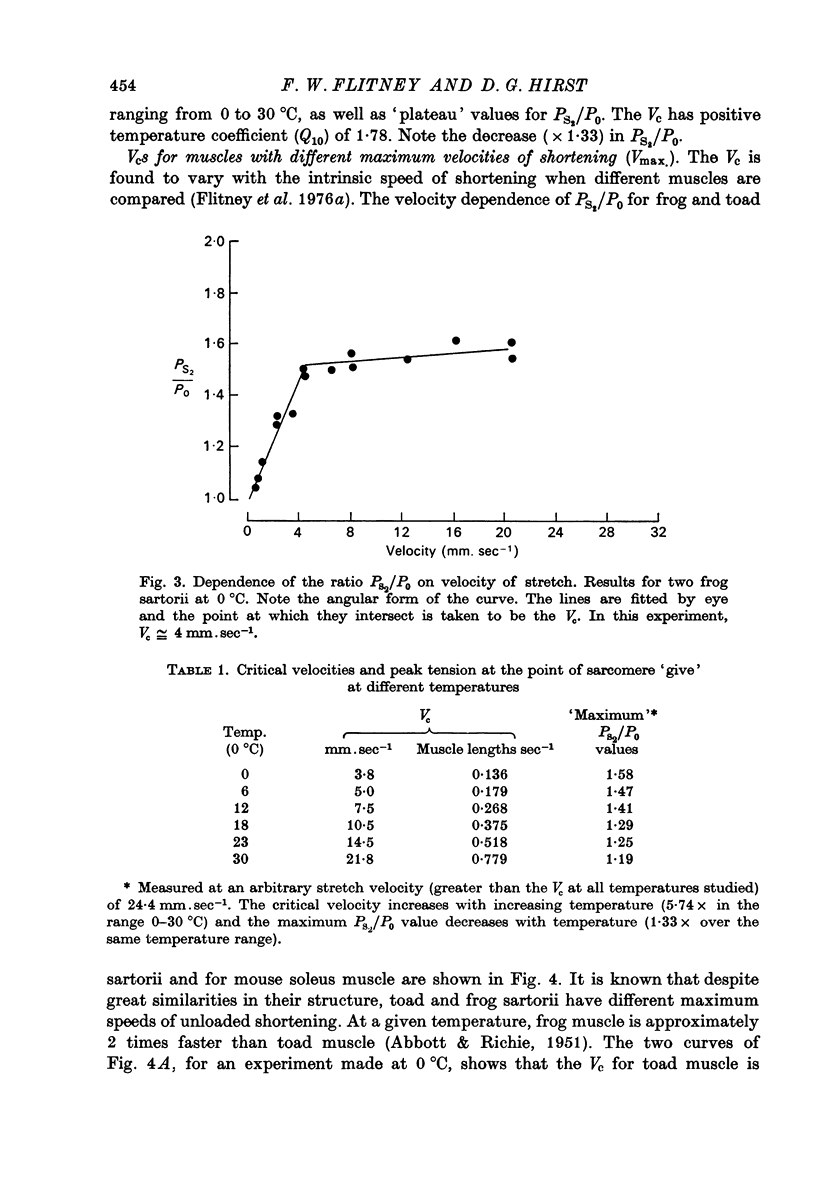
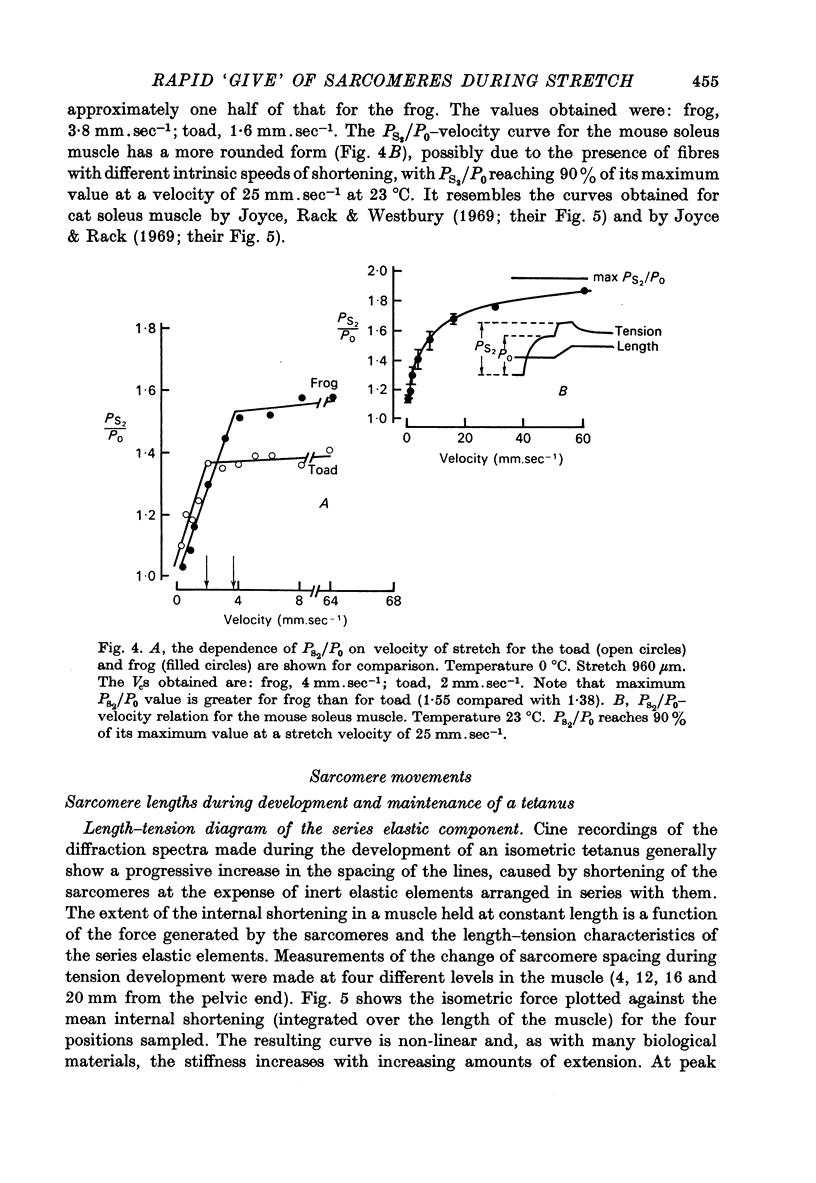
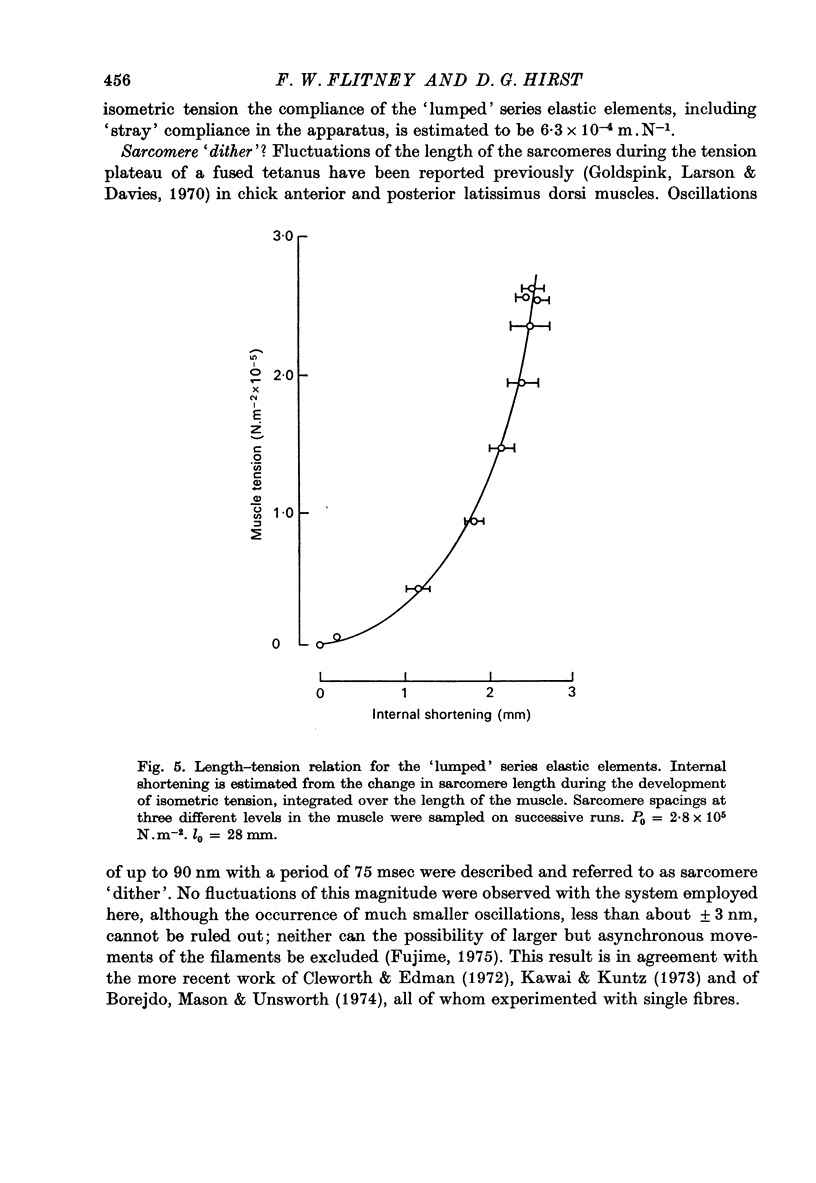
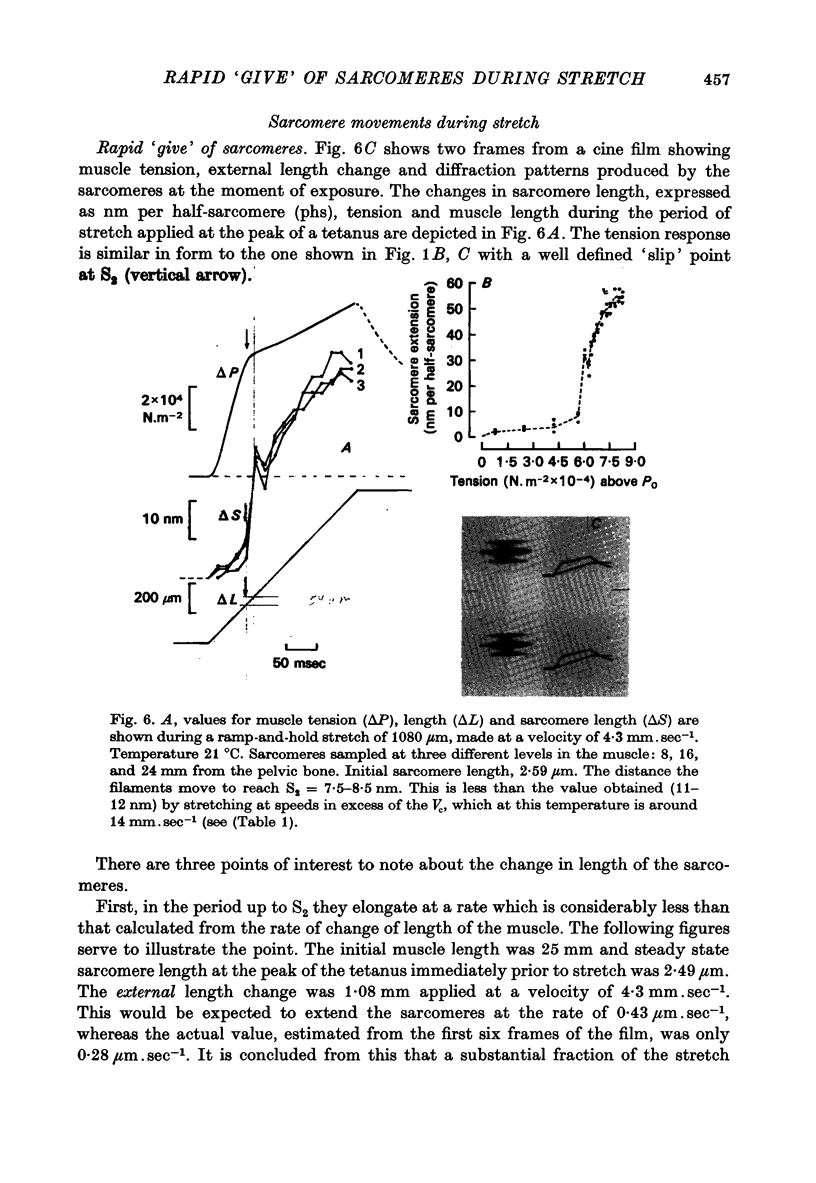
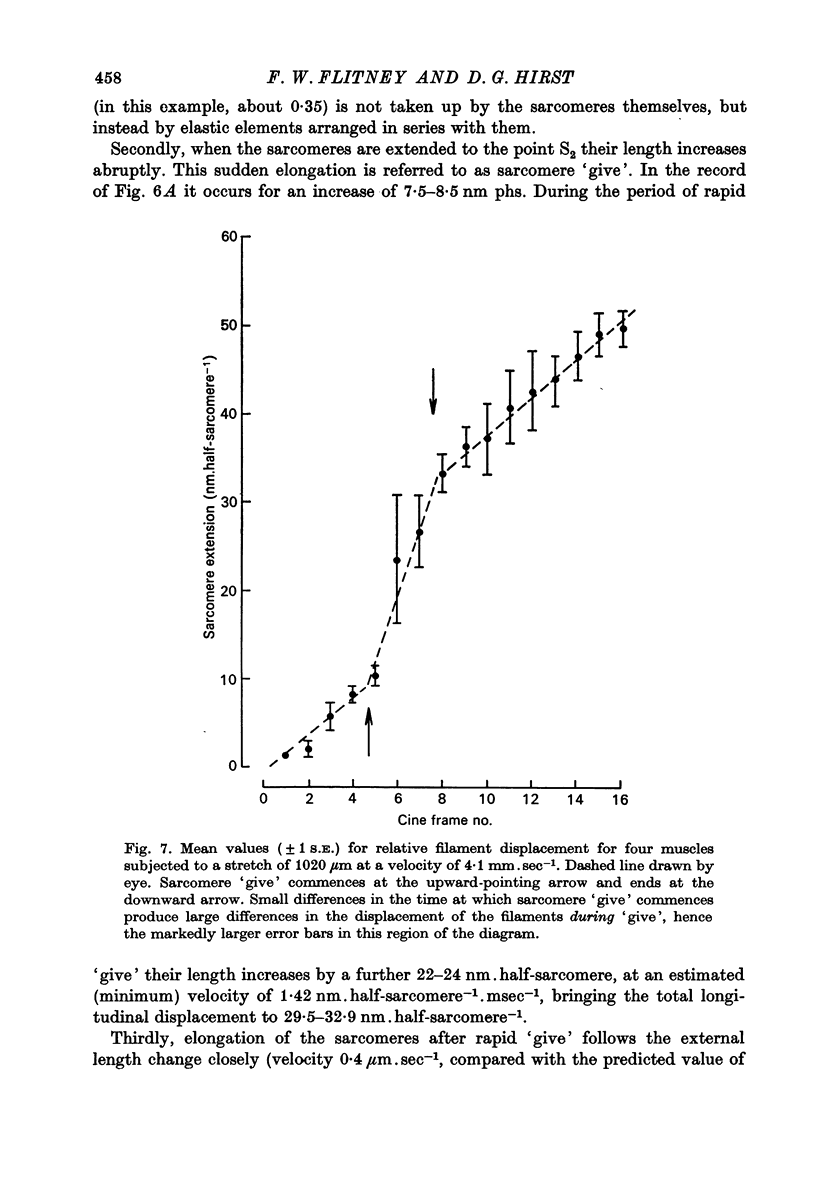
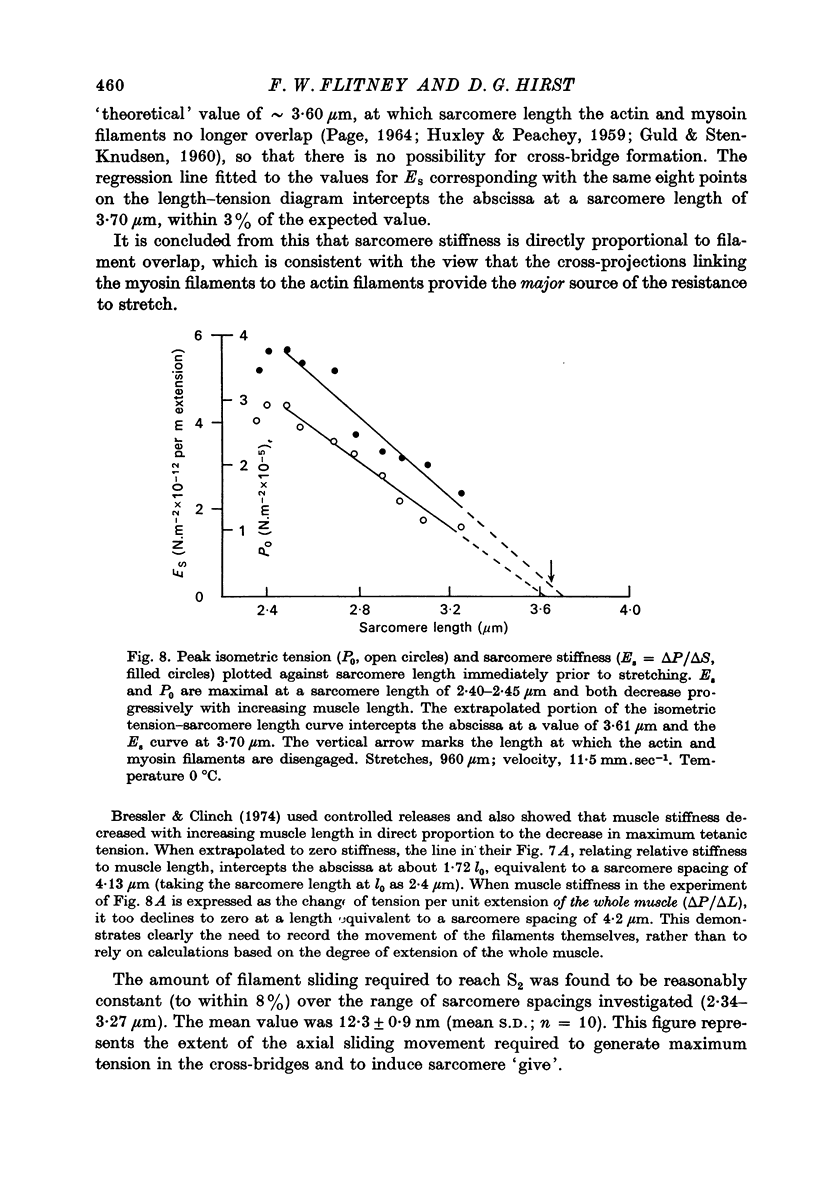
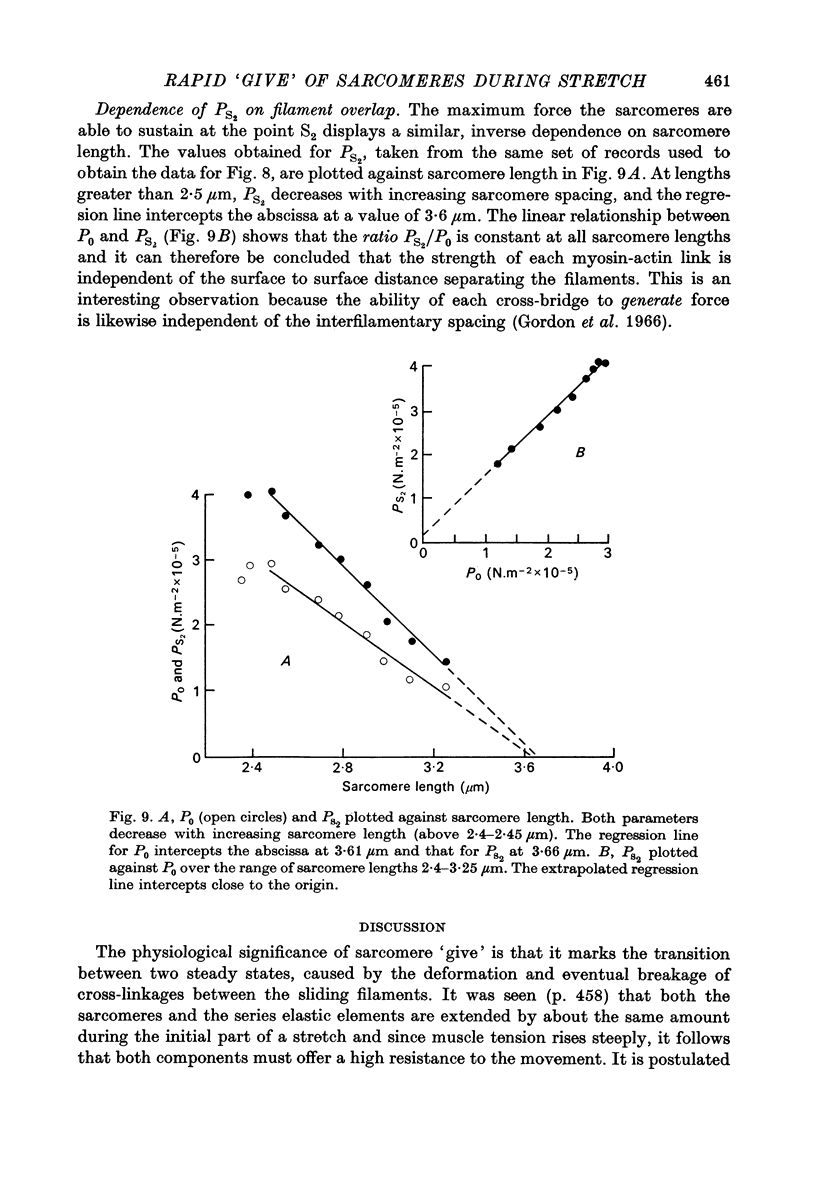
1. A study has been made of the tension responses and sarcomere length changes produced by servo-controlled stretches applied to isometrically contracting frog muscle. Sarcomere lengths were monitored by cine-photography of diffiraction spectra obtained by illuminating a small area of muscle with a laser. 2. The tension increment produced by a ramp-and-hold stretch of approximately 1 mm (ca. 4% of the muscle length) comprises three phases whose limits are defined by two points, S1 and S2, where the slope of the response decreases abruptly. S1 and S2 correspond to extensions of 0.13 and 1.2% of the muscle length. 3. Movements of the first order spectra relative to the zero order recorded during stretch reveal that S2 coincides with an abrupt elongation of the sarcomeres. This is termed sarcomere 'give' and it occurs when the filaments are displaced by 11-12 nm from their steady-state (isometric) position. 4. The stiffness of the sarcomeres, Es, up to S2 decreases with increasing sarcomere length. The maximum force sustained by the muscle at S2, PS2, also shows an inverse dependence on sarcomere length. Both Es and PS2 fall to zero at an extrapolated sarcomere spacing of 3.6-3.7 micrometer, coinciding with the length at which the actin and myosin filaments no longer overlap. 5. The ratio PS2/P0 (where P0 = maximum isometric tension) varies with temperature and speed of stretch. It increases with increasing speeds of stretch until a certain critical velocity, Vc, is reached, beyond which it is almost independent of any further increase. Vc has a positive temperature coefficient, increasing 5-6 in the range 0-30 degrees C (Q10 = 1.8). There is a positive correlation between the maximum speed of isotonic shortening (Vmax.) and Vc in different muscles. 6. Sarcomere 'give' during stretch is considered to be due to forcible detachment of cross-bridges between the actin and myosin filaments. This results in recoil of the extended series elastic elements in the muscle at the expense of the sarcomers. The amount of filament displacement required to induce sarcomere 'give' (11-12 nm) is thought to represent the range of movement over which a cross-bridge can remain attached to actin during a stretch.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT B. C., RITCHIE J. M. The onset of shortening in striated muscle. J Physiol. 1951 Apr;113(2-3):336–345. doi: 10.1113/jphysiol.1951.sp004577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borejdo J., Mason P., Unsworth J. Optical diffraction studies on stimulated single fibres of frog muscle (Hyla caerulea). Experientia. 1974 Apr 15;30(4):373–374. doi: 10.1007/BF01921671. [DOI] [PubMed] [Google Scholar]
- Bressler B. H., Clinch N. F. The compliance of contracting skeletal muscle. J Physiol. 1974 Mar;237(3):477–493. doi: 10.1113/jphysiol.1974.sp010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleworth D. R., Edman K. A. Changes in sarcomere length during isometric tension development in frog skeletal muscle. J Physiol. 1972 Dec;227(1):1–17. doi: 10.1113/jphysiol.1972.sp010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Gilbert C., Kretzschmar K. M., Wilkie D. R. The effect of the performance of work on total energy output and metabolism during muscular contraction. J Physiol. 1974 May;238(3):455–472. doi: 10.1113/jphysiol.1974.sp010537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELEZE J. B. The mechanical properties of the semitendinosus muscle at lengths greater than its length in the body. J Physiol. 1961 Sep;158:154–164. [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Elzinga G., Noble M. I. Proceedings: Force enhancement induced by stretch of contracting single isolated muscle fibres of the frog. J Physiol. 1976 Jun;258(2):95P–96P. [PubMed] [Google Scholar]
- Elliott G. F., Rome E. M., Spencer M. A type of contraction hypothesis applicable to all muscles. Nature. 1970 May 2;226(5244):417–420. doi: 10.1038/226417a0. [DOI] [PubMed] [Google Scholar]
- Flitney F. W., Hirst D. G. Filament sliding and energy absorbed by the cross-bridge in active muscle subjected to cycical length changes. J Physiol. 1978 Mar;276:467–479. doi: 10.1113/jphysiol.1978.sp012247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitney F. W., Hirst D. G. Proceedings: Tension responses and sarcomere movements during length changes applied to contracting frog's muscle. J Physiol. 1975 Sep;251(1):66P–68P. [PubMed] [Google Scholar]
- Flitney F. W., Hirst D. G., Stephens W. G. Proceedings: Apparatus for investigating sarcomere movements in contracting frog's muscle during servo-controlled length changes. J Physiol. 1975 Sep;251(1):8P–9P. [PubMed] [Google Scholar]
- Flitney F. W. Light scattering associated with tension changes in the short-range elastic component of resting frog's muscle. J Physiol. 1975 Jan;244(1):1–14. doi: 10.1113/jphysiol.1975.sp010781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujime S. Optical diffraction study of muscle fibers. Biochim Biophys Acta. 1975 Jan 30;379(1):227–238. doi: 10.1016/0005-2795(75)90026-4. [DOI] [PubMed] [Google Scholar]
- Goldspink G., Larson R. E., Davies R. E. Fluctuations in sarcomere length in the chick anterior and posterior latissimus dorsi muscles during isometric contraction. Experientia. 1970 Jan 15;26(1):16–18. doi: 10.1007/BF01900361. [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL D. K. The optical properties of resting striated muscle; the effect of rapid stretch on the scattering and diffraction of light. J Physiol. 1953 Mar;119(4):489–500. doi: 10.1113/jphysiol.1953.sp004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hill D. K. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol. 1968 Dec;199(3):637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. A capacitance-gauge tension transducer. J Physiol. 1968 Jul;197(1):12P–12P. [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M. Isotonic lengthening and shortening movements of cat soleus muscle. J Physiol. 1969 Oct;204(2):475–491. doi: 10.1113/jphysiol.1969.sp008925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M., Westbury D. R. The mechanical properties of cat soleus muscle during controlled lengthening and shortening movements. J Physiol. 1969 Oct;204(2):461–474. doi: 10.1113/jphysiol.1969.sp008924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Kuntz I. D. Optical diffraction studies of muscle fibers. Biophys J. 1973 Sep;13(9):857–876. doi: 10.1016/S0006-3495(73)86031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J. The effect of low-level activation on the mechanical properties of isolated frog muscle fibers. J Gen Physiol. 1971 Aug;58(2):145–162. doi: 10.1085/jgp.58.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Sollins M. R., Julian F. J. Calcium activation produces a characteristic response to stretch in both skeletal and cardiac muscle. Nature. 1976 Apr 15;260(5552):619–621. doi: 10.1038/260619a0. [DOI] [PubMed] [Google Scholar]
- PAGE S. FILAMENT LENGTHS IN RESTING AND EXCITED MUSCLES. Proc R Soc Lond B Biol Sci. 1964 Oct 27;160:460–466. doi: 10.1098/rspb.1964.0056. [DOI] [PubMed] [Google Scholar]
- Rack P. M., Westbury D. R. The short range stiffness of active mammalian muscle and its effect on mechanical properties. J Physiol. 1974 Jul;240(2):331–350. doi: 10.1113/jphysiol.1974.sp010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear D. B. Electrostatic forces in muscle contraction. J Theor Biol. 1970 Sep;28(3):531–546. doi: 10.1016/0022-5193(70)90087-1. [DOI] [PubMed] [Google Scholar]
- Sugi H. Tension changes during and after stretch in frog muscle fibres. J Physiol. 1972 Aug;225(1):237–253. doi: 10.1113/jphysiol.1972.sp009935. [DOI] [PMC free article] [PubMed] [Google Scholar]



