Abstract
1. Synapses by flexor nerve were induced on denervated extensor muscle in adult salamander forelimbs. Excitatory potentials evoked by these `foreign' synapses were at first small but increased to normal amplitude within several weeks, in the absence of correct nerve reinnervation.
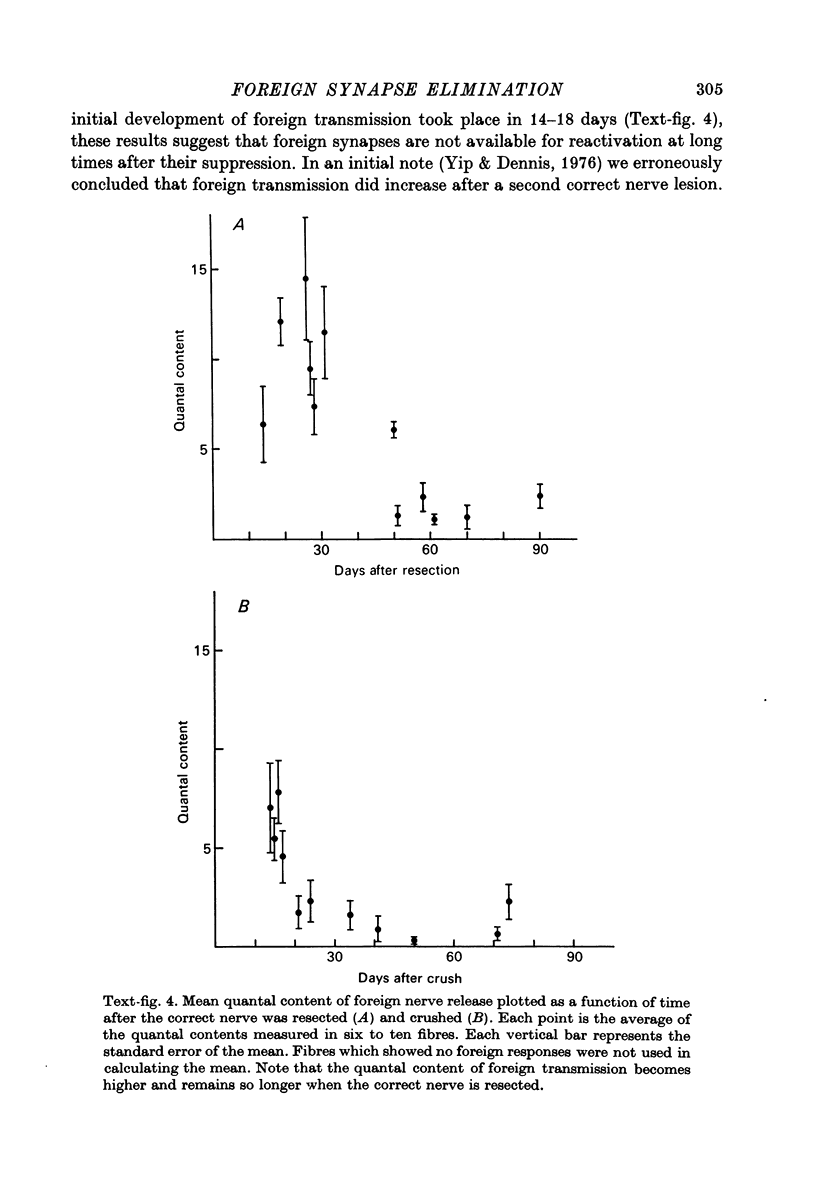
2. Upon return of the correct nerve the efficacy of foreign synaptic transmission began to decline. The time of initiation of this decline correlated well with the resumption of correct nerve transmission. The suppression of foreign transmission involved a reduction in mean quantal content of transmitter release.
3. Suppression of foreign synapses was sufficiently thorough that most ceased transmitting entirely. Before reinnervation by the correct nerve 97% of the extensor muscle fibres received functional foreign synapses while 4-6 months after correct nerve return only 35% of the fibres retained foreign synapses, with weak transmission.
4. Two lines of evidence indicate that suppressed foreign synapses are lost from the muscle: (a) a second correct nerve lesion 6-8 months after the initial denervation produced no significant increase in the proportion of fibres with foreign transmission and (b) four muscles which showed complete suppression of foreign transmission were bathed in medium containing horseradish peroxidase (h.r.p.) and the correct nerve was stimulated repetitively. Subsequent histochemical staining for h.r.p. and examination of synapses by electron microscopy revealed that 94% of the axon terminals had h.r.p. incorporated in vesicles. Thus at least that percentage of all identifiable synapses were from the correct nerve.
5. This ability to eliminate incorrect synapses in favour of correct ones is speculated to be a general characteristic of embryonic nervous systems, which in adulthood is retained by salamanders but lost by most other animals.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNSTEIN J. J., GUTH L. Nonselectivity in establishment of neuromuscular connections following nerve regeneration in the rat. Exp Neurol. 1961 Sep;4:262–275. doi: 10.1016/0014-4886(61)90047-4. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass D. T., Sutton T. J., Mark R. F. Competition between nerves for functional connexions with axolotl muscles. Nature. 1973 May 25;243(5404):201–203. doi: 10.1038/243201a0. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Non-transmitting neuromuscular junctions during an early stage of end-plate reinnervation. J Physiol. 1974 Jun;239(3):553–570. doi: 10.1113/jphysiol.1974.sp010582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J. Physiological properties of junctions between nerve and muscle developing during salamander limb regeneration. J Physiol. 1975 Jan;244(3):683–702. doi: 10.1113/jphysiol.1975.sp010820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangboner R. F., Vanable J. W., Jr Formation and regression of inappropriate nerve sprouts during trochlear nerve regeneration in Xenopus laevis. J Comp Neurol. 1974 Oct 15;157(4):391–406. doi: 10.1002/cne.901570404. [DOI] [PubMed] [Google Scholar]
- Frank E., Jansen J. K. Interaction between foreign and original nerves innervating gill muscles in fish. J Neurophysiol. 1976 Jan;39(1):84–90. doi: 10.1152/jn.1976.39.1.84. [DOI] [PubMed] [Google Scholar]
- Frank E., Jansen J. K., Lomo T., Westgaard R. H. The interaction between foreign and original motor nerves innervating the soleus muscle of rats. J Physiol. 1975 Jun;247(3):725–743. doi: 10.1113/jphysiol.1975.sp010954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Grimm L. M. An evaluation of myotypic respecification in axolotls. J Exp Zool. 1971 Dec;178(4):479–496. doi: 10.1002/jez.1401780406. [DOI] [PubMed] [Google Scholar]
- Grinnell A. D., Rheuben M. B., Letinsky M. S. Mutual repression of synaptic efficacy by pairs of foreign nerves innervating frog skeletal muscle. Nature. 1977 Jan 27;265(5592):368–370. doi: 10.1038/265368a0. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh J. F. Selective reinnervation of fast-twitch and slow-graded muscle fibers in the toad. Exp Neurol. 1971 Feb;30(2):263–276. doi: 10.1016/s0014-4886(71)80006-7. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneliussen H., Jansen J. K. Morphological aspects of the elimination of polyneuronal innervation of skeletal muscle fibres in newborn rats. J Neurocytol. 1976 Oct;5(8):591–604. doi: 10.1007/BF01175572. [DOI] [PubMed] [Google Scholar]
- Lehouelleur J., Chatelain A. Analysis of electrical responses of newt skeletal muscle fibres in response to direct and indirect stimulation. J Physiol (Paris) 1974;68(6):615–632. [PubMed] [Google Scholar]
- Lentz T. L. Development of the neuromuscular junction. I. Cytological and cytochemical studies on the neuromuscular junction of differentiating muscle in the regenerating limb of the newt Triturus. J Cell Biol. 1969 Aug;42(2):431–443. doi: 10.1083/jcb.42.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark R. F., Marotte L. R., Mart P. E. The mechanism of selective reinnervation of fish eye muscles. IV. Identification of repressed synapses. Brain Res. 1972 Nov 13;46:149–157. doi: 10.1016/0006-8993(72)90012-1. [DOI] [PubMed] [Google Scholar]
- Mark R. F., Marotte L. R. The mechanism of selective reinnervation of fish eye muscles. 3. Functional, electrophysiological and anatomical analysis of recovery from section of 3rd and IVth nerves. Brain Res. 1972 Nov 13;46:131–148. doi: 10.1016/0006-8993(72)90011-x. [DOI] [PubMed] [Google Scholar]
- Marotte L. R., Mark R. F. The mechanism of selective reinnervation of fish eye muscle. I. Evidence from muscle function during recovery. Brain Res. 1970 Apr 1;19(1):41–51. doi: 10.1016/0006-8993(70)90235-0. [DOI] [PubMed] [Google Scholar]
- Marotte L. R., Mark R. P. The mechanism of selective reinnervation of fish eye muscle. II. Evidence from electronmicroscopy of nerve endings. Brain Res. 1970 Apr 1;19(1):53–62. doi: 10.1016/0006-8993(70)90236-2. [DOI] [PubMed] [Google Scholar]
- Schmidt H., Stefani E. Re-innervation of twitch and slow muscle fibres of the frog after crushing the motor nerves. J Physiol. 1976 Jun;258(1):99–123. doi: 10.1113/jphysiol.1976.sp011409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S. A. Persistence of foreign innervation on reinnervated goldfish extraocular muscles. Science. 1975 Aug 22;189(4203):644–646. doi: 10.1126/science.1162348. [DOI] [PubMed] [Google Scholar]
- Yip J. W., Dennis M. J. Suppression of transmission at foreign synapses in adult newt muscle involves reduction in quantal content. Nature. 1976 Mar 25;260(5549):350–352. doi: 10.1038/260350a0. [DOI] [PubMed] [Google Scholar]