Abstract
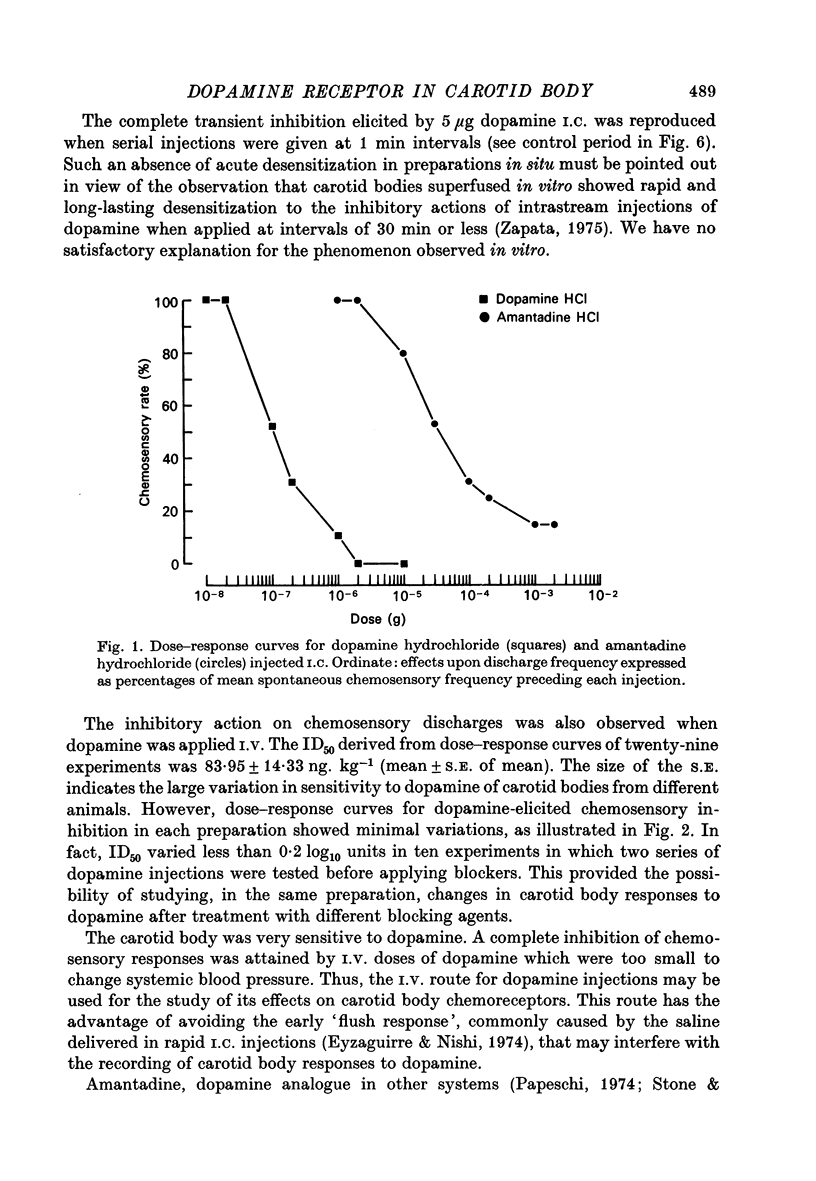
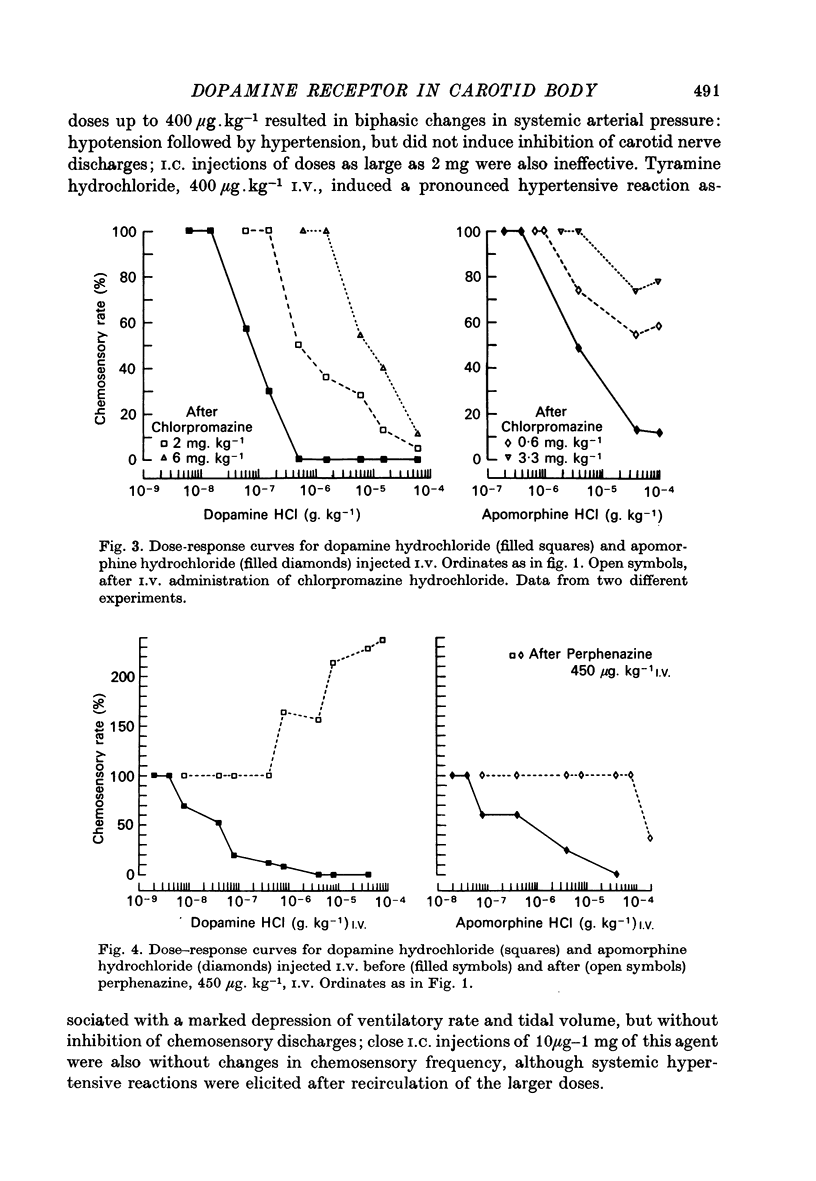
1. The effects of dopamine, its analogues and antagonists on the chemosensory discharges originating from carotid bodies in situ were studied in anaesthetized cats. 2. Intracarotid (I.C.) injections of 100 ng or more of dopamine produced transient depression of the frequency of carotid nerve chemosensory discharges. Short term (1-5 sec) complete inhibition was usually elicited by 2 microgram dopamine. 3. I.V. injections of dopamine also produced inhibition of chemosensory discharges, an effect observed with doses which were still too low to produce changes in systemic arterial pressure. Half-maximal inhibition (ID50) of chemoreceptors was elicited with a mean dose of 84 ng.kg-1. 4. I.C. and I.V. injections of apomorphine and amantadine also produced transient inhibition of chemosensory activity. Higher doses of these analogues of dopamine were needed to produce this effect, and the resulting inhibition usually did not silence the nerve discharges. Apomorphine inhibition was slightly more prolonged than that with dopamine. 5. Large doses of amphetamine and tyramine, inducers of dopamine release, did not produce inhibition of chemosensory discharges. 6. The effects of two classes of dopamine antagonists were tested. Dose-response curves for dopamine and apomorphine inhibition were displaced to the right by administration of phenothiazines (chlorpromazine and perphenazine) and butyrophenones (haloperidol and spiroperidol). In animals treated with perphenazine or spiroperidol, dopamine became a stimulator of chemoreceptor activity. 7. It is suggested that dopamine present in carotid body may operate as a modulator of chemosensory activity.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biscoe T. J., Purves M. J. Observations on the rhythmic variation in the cat carotid body chemoreceptor activity which has the same period as respiration. J Physiol. 1967 Jun;190(3):389–412. doi: 10.1113/jphysiol.1967.sp008217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Makman M. H. Influence of neuroleptic drugs and apomorphine on dopamine-sensitive adenylate cyclase of retina. J Neurochem. 1973 Aug;21(2):477–479. doi: 10.1111/j.1471-4159.1973.tb04268.x. [DOI] [PubMed] [Google Scholar]
- Bunney B. S., Walters J. R., Roth R. H., Aghajanian G. K. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 1973 Jun;185(3):560–571. [PubMed] [Google Scholar]
- Carlsson A., Fuxe K., Hamberger B., Lindqvist M. Biochemical and histochemical studies on the effects of imipramine-like drugs and (+)-amphetamine on central and peripheral catecholamine neurons. Acta Physiol Scand. 1966 Jul-Aug;67(3):481–497. doi: 10.1111/j.1748-1716.1966.tb03334.x. [DOI] [PubMed] [Google Scholar]
- Clement-Cormier Y. C., Kebabian J. W., Petzold G. L., Greengard P. Dopamine-sensitive adenylate cyclase in mammalian brain: a possible site of action of antipsychotic drugs. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1113–1117. doi: 10.1073/pnas.71.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools A. R., Van Rossum J. M. Excitation-mediating and inhibition-mediating dopamine-receptors: a new concept towards a better understanding of electrophysiological, biochemical, pharmacological, functional and clinical data. Psychopharmacologia. 1976 Feb 2;45(3):243–254. doi: 10.1007/BF00421135. [DOI] [PubMed] [Google Scholar]
- Ernst A. M. The role of biogenic amines in the extra-pyramidal system. Acta Physiol Pharmacol Neerl. 1969 Jun;15(2):141–154. [PubMed] [Google Scholar]
- Eyzaguirre C., Nishi K. Further study on mass receptor potential of carotid body chemosensors. J Neurophysiol. 1974 Jan;37(1):156–169. doi: 10.1152/jn.1974.37.1.156. [DOI] [PubMed] [Google Scholar]
- Hellström S., Hanbauer I., Costa E. Selective decrease of dopamine content in rat carotid body during exposure to hypoxic conditions. Brain Res. 1976 Dec 17;118(2):352–355. doi: 10.1016/0006-8993(76)90725-3. [DOI] [PubMed] [Google Scholar]
- Jackson D. M., Andén N. E., Dahlström A. A functional effect of dopamine in the nucleus accumbens and in some other dopamine-rich parts of the rat brain. Psychopharmacologia. 1975 Dec 31;45(2):139–149. doi: 10.1007/BF00429052. [DOI] [PubMed] [Google Scholar]
- Jacobs L., Comroe J. H., Jr Stimulation of the carotid chemoreceptors of the dog by dopamine. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1187–1193. doi: 10.1073/pnas.59.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen P. A., Niemegeers C. J., Schellekens K. H., Lenaerts F. M. Is it possible to predict the clinical effects of neuroleptic drugs (major tranquillizers) from animal data? IV. An improved experimental design for measuring the inhibitory effects of neuroleptic drugs on amphetamine-or apomorphine-induced "Cheroing" and "agitation" in rats. Arzneimittelforschung. 1967 Jul;17(7):841–854. [PubMed] [Google Scholar]
- Kobayashi S. Comparative cytological studies of the carotid body. 1. Demonstration of monoamine-storing cells by correlated chromaffin reaction and fluorescence histochemistry. Arch Histol Jpn. 1971 Oct;33(4):319–339. [PubMed] [Google Scholar]
- Libet B., Tosaka T. Dopamine as a synaptic transmitter and modulator in sympathetic ganglia: a different mode of synaptic action. Proc Natl Acad Sci U S A. 1970 Oct;67(2):667–673. doi: 10.1073/pnas.67.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan H., York D. H. The action of dopamine on neurones of the caudate nucleus. J Physiol. 1967 Apr;189(3):393–402. doi: 10.1113/jphysiol.1967.sp008175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E., Slotkin T. A., Sampson S. R. Letter: Carotid body chemoreceptors. Nature. 1975 Nov 20;258(5532):268–269. doi: 10.1038/258268b0. [DOI] [PubMed] [Google Scholar]
- Osborne M. P., Butlar P. J. New theory for receptor mechanism of carotid body chemoreceptors. Nature. 1975 Apr 24;254(5502):701–703. doi: 10.1038/254701a0. [DOI] [PubMed] [Google Scholar]
- Papeschi R. Amantadine may stimulate dopamine and noradrenaline receptors. Neuropharmacology. 1974 Jan;13(1):77–83. doi: 10.1016/0028-3908(74)90009-4. [DOI] [PubMed] [Google Scholar]
- Sampson S. R., Aminoff M. J., Jaffe R. A., Vidruk E. H. Analysis of inhibitory effect of dopamine on carotid body chemoreceptors in cats. Am J Physiol. 1976 Jun;230(6):1494–1498. doi: 10.1152/ajplegacy.1976.230.6.1494. [DOI] [PubMed] [Google Scholar]
- Simon A., Van Maanen E. F. Dopamine receptors and dopaminergic nerves in the vas deferens of the rat. Arch Int Pharmacodyn Ther. 1976 Jul;222(1):4–15. [PubMed] [Google Scholar]
- Stone T. W., Bailey E. V. Responses of central neurones to amantadine: comparison with dopamine and amphetamine. Brain Res. 1975 Feb 21;85(1):126–129. doi: 10.1016/0006-8993(75)91017-3. [DOI] [PubMed] [Google Scholar]
- Von Voigtlander P. F., Moore K. E. Involvement of nigro-striatal neurons in the in vivo release of dopamine by amphetamine, amantadine and tyramine. J Pharmacol Exp Ther. 1973 Mar;184(3):542–552. [PubMed] [Google Scholar]
- Willems J. L. Dopamine-induced inhibition of synaptic transmission in lumbar paravertebral ganglia of the dog. Naunyn Schmiedebergs Arch Pharmacol. 1973;279(2):115–126. doi: 10.1007/BF00503977. [DOI] [PubMed] [Google Scholar]
- York D. H. Dopamine receptor blockade--a central action of chlorpromazine on striatal neurones. Brain Res. 1972 Feb 11;37(1):91–99. doi: 10.1016/0006-8993(72)90348-4. [DOI] [PubMed] [Google Scholar]
- Zapata P. Effects of dopamine on carotid chemo- and baroreceptors in vitro. J Physiol. 1975 Jan;244(1):235–251. doi: 10.1113/jphysiol.1975.sp010794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata P., Hess A., Bliss E. L., Eyzaguirre C. Chemical, electron microscopic and physiological observations on the role of catecholamines in the carotid body. Brain Res. 1969 Jul;14(2):473–496. doi: 10.1016/0006-8993(69)90123-1. [DOI] [PubMed] [Google Scholar]
- Zapata P. Modulatory role of dopamine on arterial chemoreceptors. Adv Biochem Psychopharmacol. 1977;16:291–298. [PubMed] [Google Scholar]


