Abstract
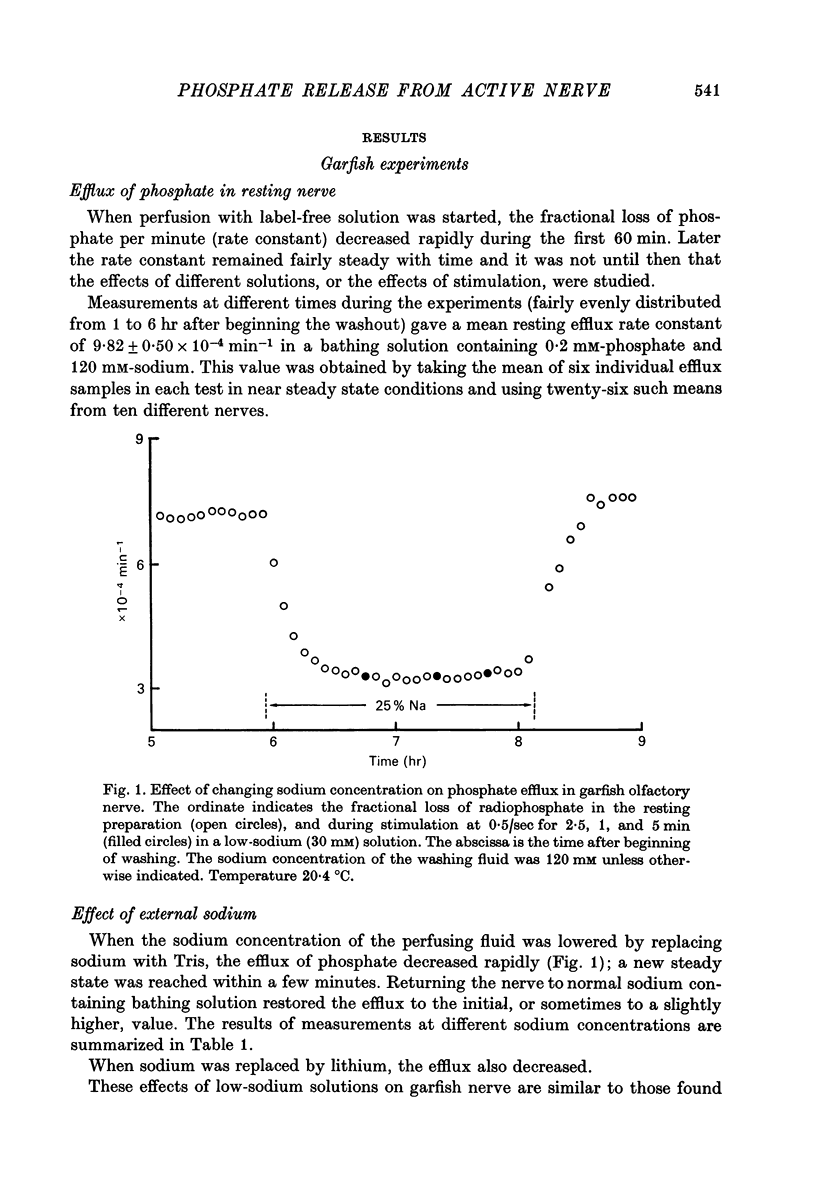
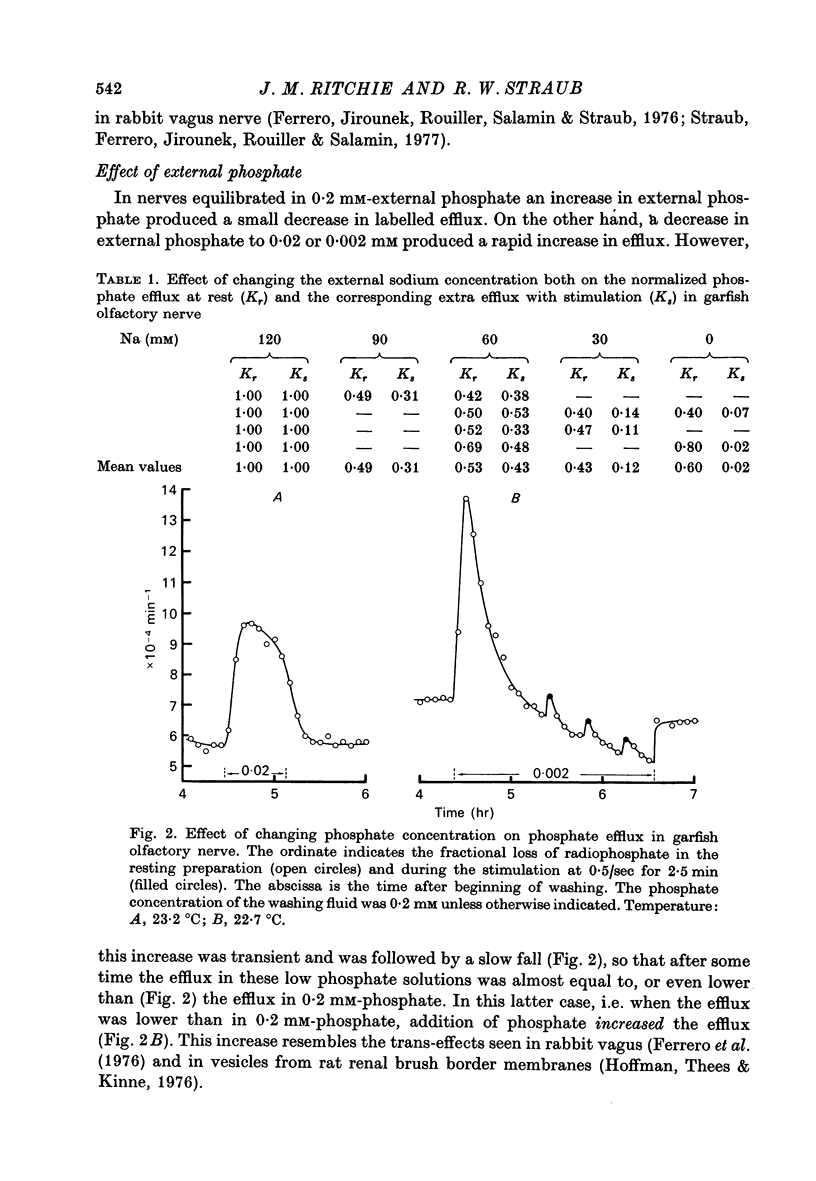
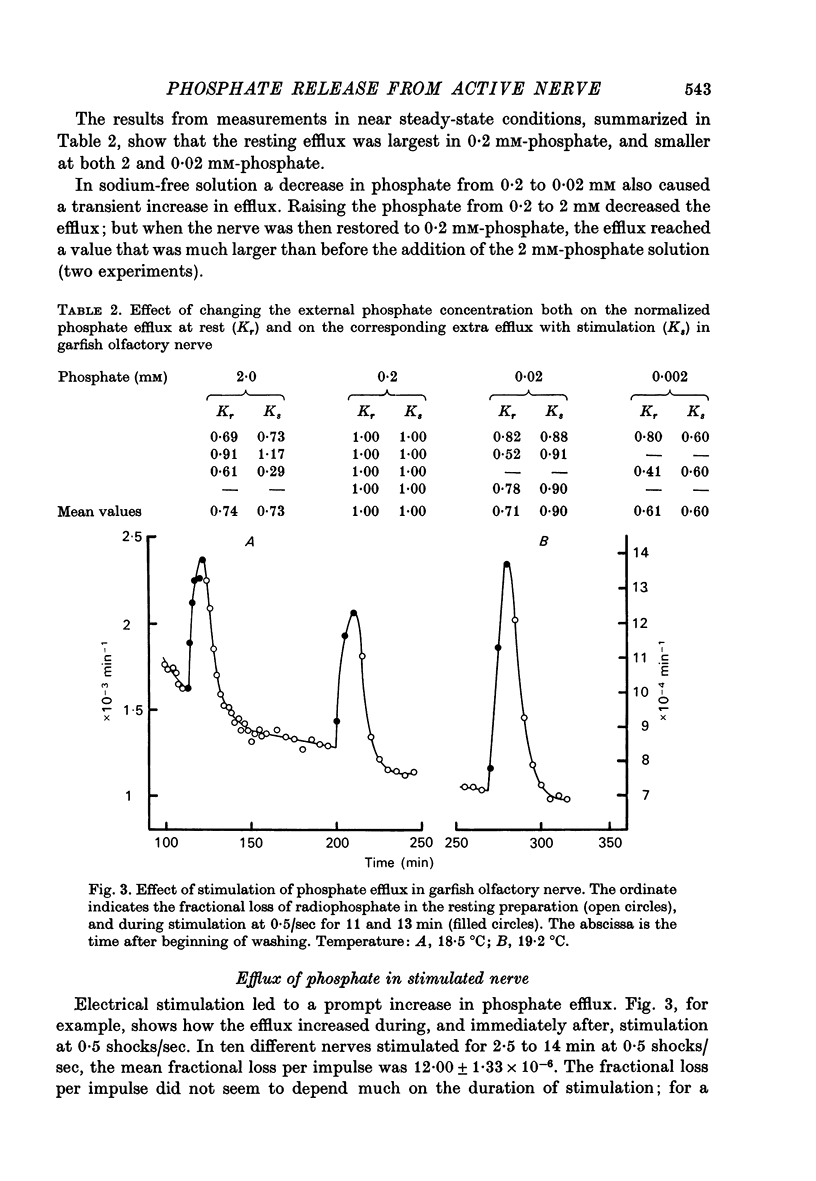
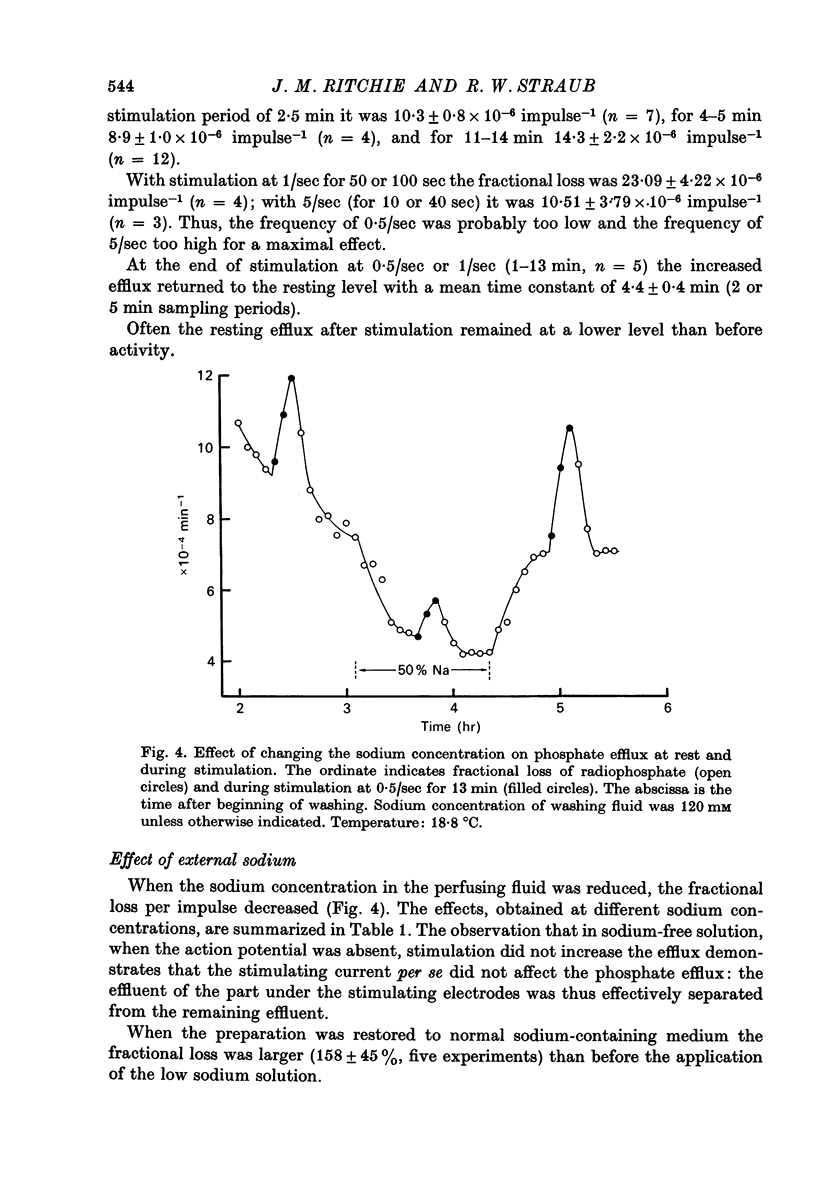
1. The movements of labelled phosphate were measured in garfish olfactory and in rabbit vagus nerves at rest and during activity. 2. In garfish olfactory nerve kept in solutions with 120 mM-sodium and 0.2 mM-phosphate the fractional loss of 32P was 9.82 X 10(-4) min-1. Lowering the sodium concentration of the washing fluid decreased the efflux; lowering the phosphate produced a transient increase with subsequent return towards the efflux in 0.2 mM-phosphate. 3. Stimulation at 0.50 sec produced an extra fractional loss of 12 X 10(-6) impulse-1. At 1/sec the effect was larger; at 5/sec it was about the same as at 0.5/sec. 4. After stimulation the effect of activity disappeared exponentially with a time constant of 4.4 min. 5. Lowering the sodium decreased the extra efflux with stimulation, whereas changing the phosphate concentration did not much affect the extra efflux. 6. In rabbit vagus nerve kept in 154 mM-sodium and 0.2 M-phosphate the fractional loss of 32P was 4.91 X 10(-4) min-1. Lowering the sodium or the phosphate decreased the resting efflux. 7. Stimulation of the vagus nerve at 15/sec produced an extra fractional loss of 0.87 X 10(-6) impulse-1. 8. The extra efflux with stimulation seems to result predominantly from an increase in intracellular inorganic phosphate resulting from increased break-down of ATP after activity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABOOD L. G., KOKETSU K., MIYAMOTO S. Outflux of various phosphates during membrane depolarization of excitable tissues. Am J Physiol. 1962 Mar;202:469–474. doi: 10.1152/ajplegacy.1962.202.3.469. [DOI] [PubMed] [Google Scholar]
- Anner B., Ferrero J., Jirounek P., Jones G. J., Salamin A., Straub R. W. Sodium-dependent influx of orthophosphate in mammalian non-myelinated nerve. J Physiol. 1976 Sep;260(3):667–686. doi: 10.1113/jphysiol.1976.sp011538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anner B., Ferrero J., Jirounek P., Straub R. W. Uptake of orthophosphate by rabbit vagus nerve fibres. J Physiol. 1975 Jun;247(3):759–771. doi: 10.1113/jphysiol.1975.sp010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Phosphorus metabolism of intact crab nerve and its relation to the active transport of ions. J Physiol. 1965 Sep;180(2):383–423. doi: 10.1113/jphysiol.1965.sp007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmouliovsky M., Schorderet M., Straub R. W. Effect of electrical activity on the concentration of phosphorylated metabolites and inorganic phosphate in mammalian non-myelinated nerve fibres. J Physiol. 1969 Jun;202(2):90P–92P. [PubMed] [Google Scholar]
- Easton D. M. Garfish olfactory nerve: easily accessible source of numerous long, homogeneous, nonmyelinated axons. Science. 1971 May 28;172(3986):952–955. doi: 10.1126/science.172.3986.952. [DOI] [PubMed] [Google Scholar]
- Ferrero J., Jirounek P., Rouiller M., Salamin A., Straub R. W. Efflux of inorganic phosphate from rabbit vagus in Locke and Na-free Locke [proceedings]. J Physiol. 1976 Dec;263(1):215P–216P. [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. Effect of frequency of electrical stimulation on the concentration of intermediary metabolites in mammalian non-myelinated fibres. J Physiol. 1959 Oct;148:353–361. doi: 10.1113/jphysiol.1959.sp006292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann N., Thees M., Kinne R. Phosphate transport by isolated renal brush border vesicles. Pflugers Arch. 1976 Mar 30;362(2):147–156. doi: 10.1007/BF00583641. [DOI] [PubMed] [Google Scholar]
- Howarth J. V., Keynes R. D., Ritchie J. M., von Muralt A. The heat production associated with the passage of a single impulse in pike olfactory nerve fibres. J Physiol. 1975 Jul;249(2):349–368. doi: 10.1113/jphysiol.1975.sp011019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLINS L. J. Phosphate exchange in nerve. J Cell Physiol. 1954 Aug;44(1):77–86. doi: 10.1002/jcp.1030440107. [DOI] [PubMed] [Google Scholar]
- McDougal D. B., Osborn L. A. Post-tetanic hyperpolarization, sodium-potassium-activated adenosine triphosphatase and high energy phosphate levels in garfish olfactory nerve. J Physiol. 1976 Mar;256(1):41–60. doi: 10.1113/jphysiol.1976.sp011310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. The ionic content of mammalian non-myelinated nerve fibres and its alteration as a result of electrical activity. J Physiol. 1968 May;196(1):223–236. doi: 10.1113/jphysiol.1968.sp008503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M. Energetic aspects of nerve conduction: the relationships between heat production, electrical activity and metabolism. Prog Biophys Mol Biol. 1973;26:147–187. doi: 10.1016/0079-6107(73)90019-9. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Straub R. W. The movement of potassium ions during electrical activity, and the kinetics of the recovery process, in the non-myelinated fibres of the garfish olfactory nerve. J Physiol. 1975 Jul;249(2):327–348. doi: 10.1113/jphysiol.1975.sp011018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Hubbard J. I. Thermal synthesis of amino acids from a simulated primitive atmosphere. Nature. 1973 Jun 15;243(5407):404–405. doi: 10.1038/243404a0. [DOI] [PubMed] [Google Scholar]
- Straub R. W., Ferrero J., Jirounek P., Rouiller M., Salamin A. Sodium-dependent transport of orthophosphate in nerve fibres. Adv Exp Med Biol. 1977;81:333–344. doi: 10.1007/978-1-4613-4217-5_33. [DOI] [PubMed] [Google Scholar]


