Abstract
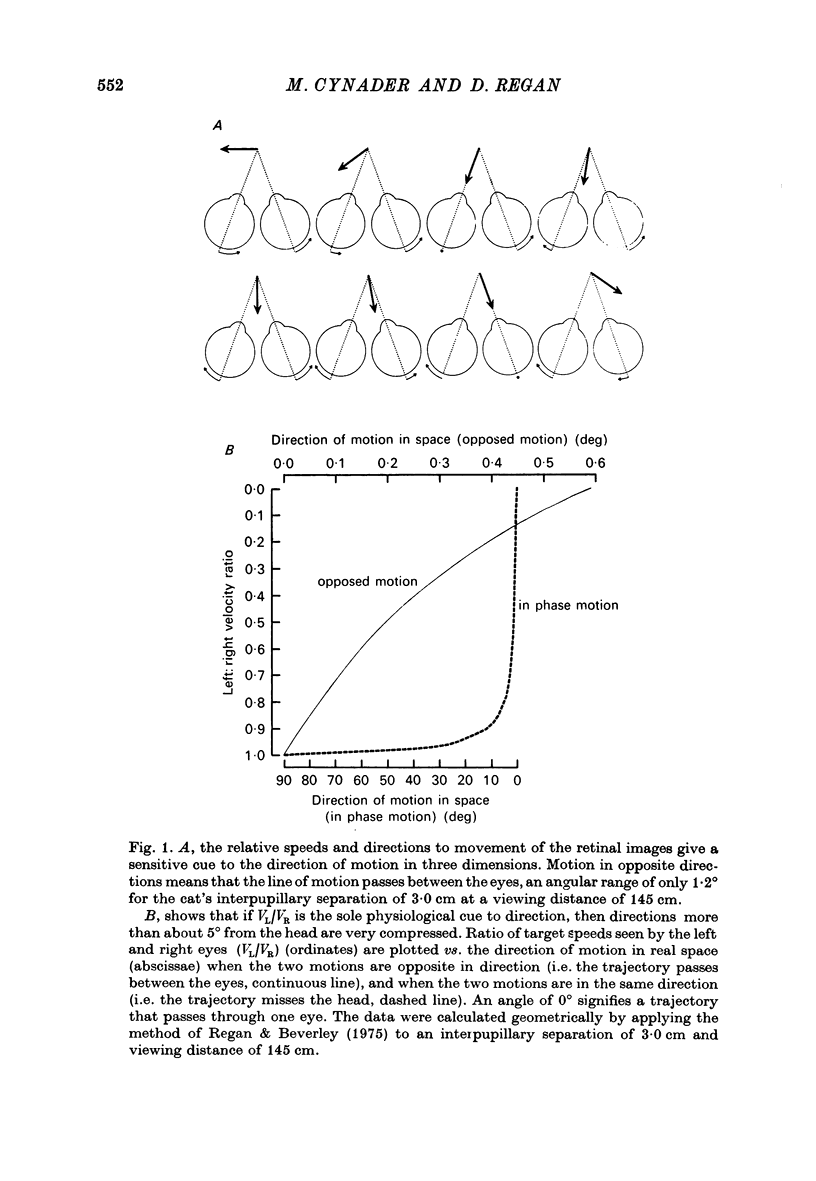
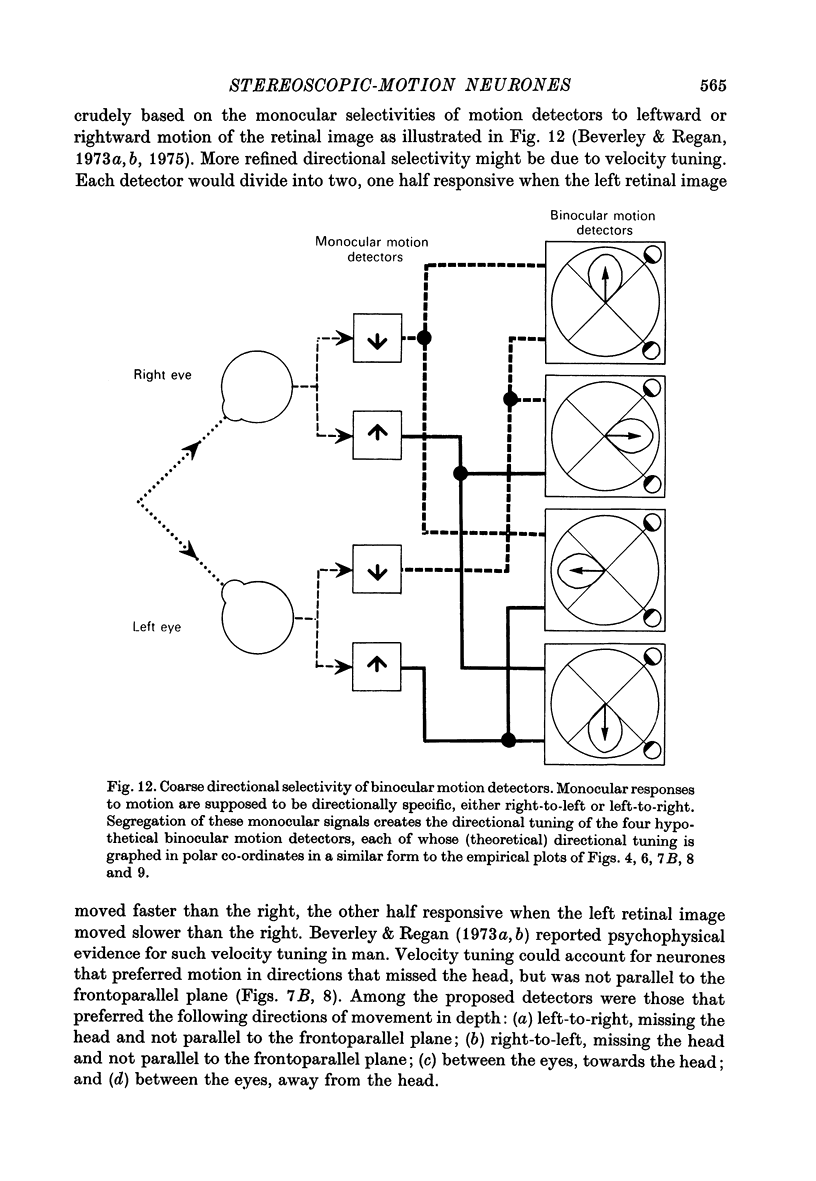
1. On psychophysical grounds, Beverley & Regan suggested that in man different neural mechanisms mediate the binocular perception of movement in depth and the binocular perception of positional (static) depth. They proposed that the human visual pathway contains several neural mechanisms, each sensitive to a different direction of motion in space. These mechanisms compute the direction of motion from the relative speeds and directions of movement of the left and right retinal images.
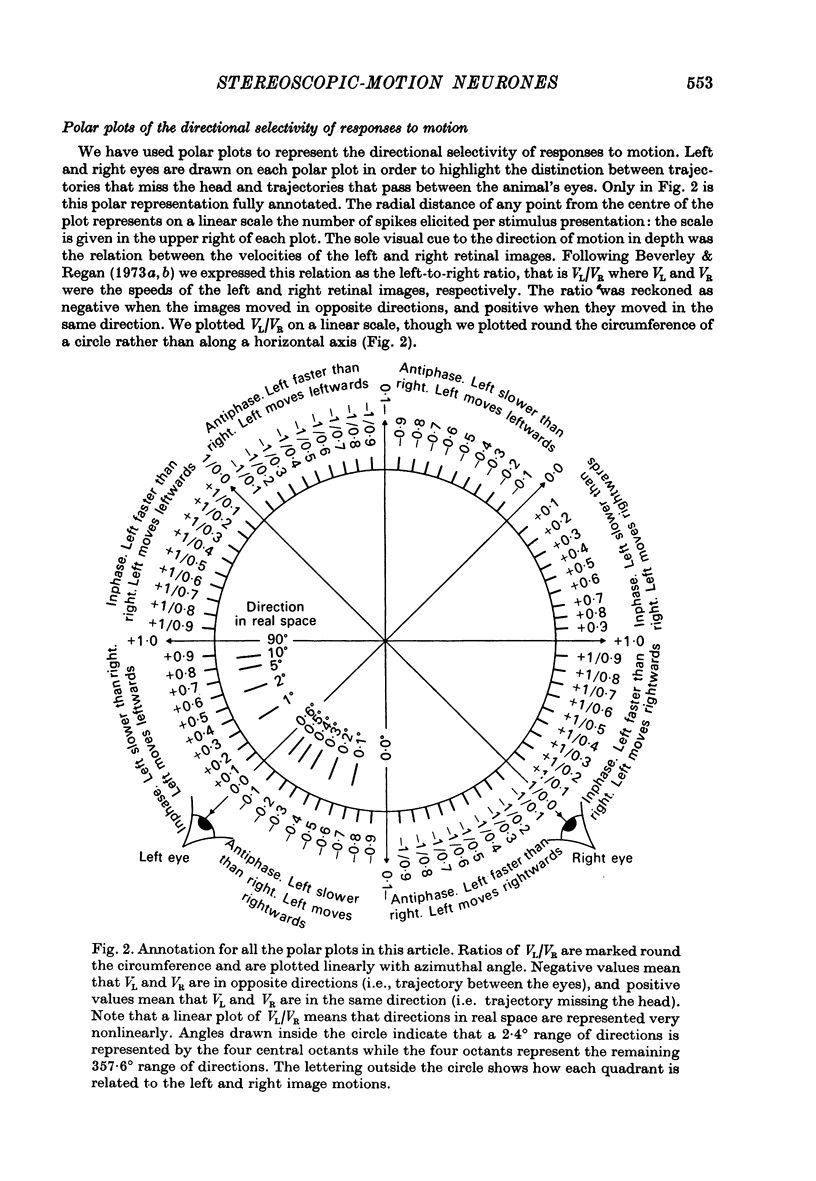
2. We have recorded from 101 units in area 18 of cat visual cortex, searching for neurones tuned to the direction of motion in three dimensions, with properties that could account for the proposed directionally tuned binocular motion detectors in man. The cat's left eye viewed one bar, while its right eye simultaneously viewed a second bar. Single units were stimulated by independently oscillating the bars from side to side. The apparent direction of movement in three dimensions was altered by varying the relative speeds of the bars and their relative directions of motion. The mean (positional) disparity of the bars could also be varied.
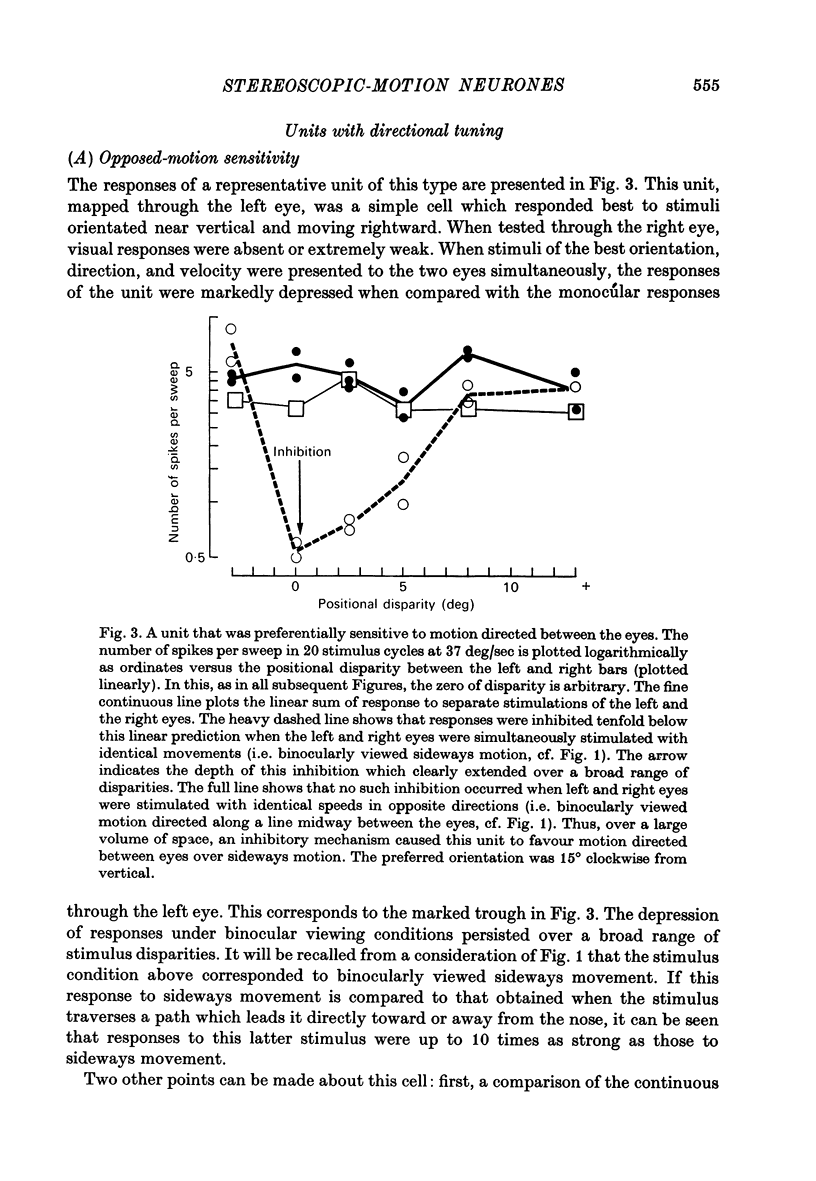
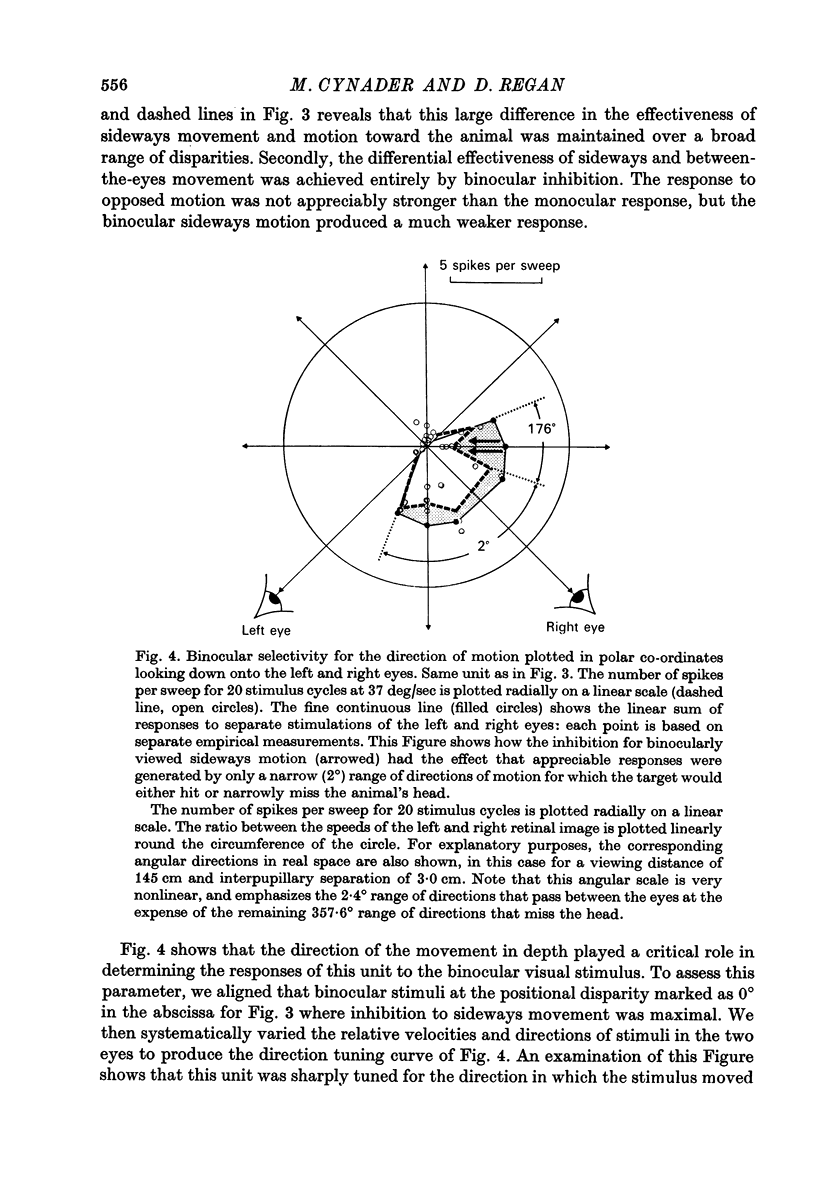
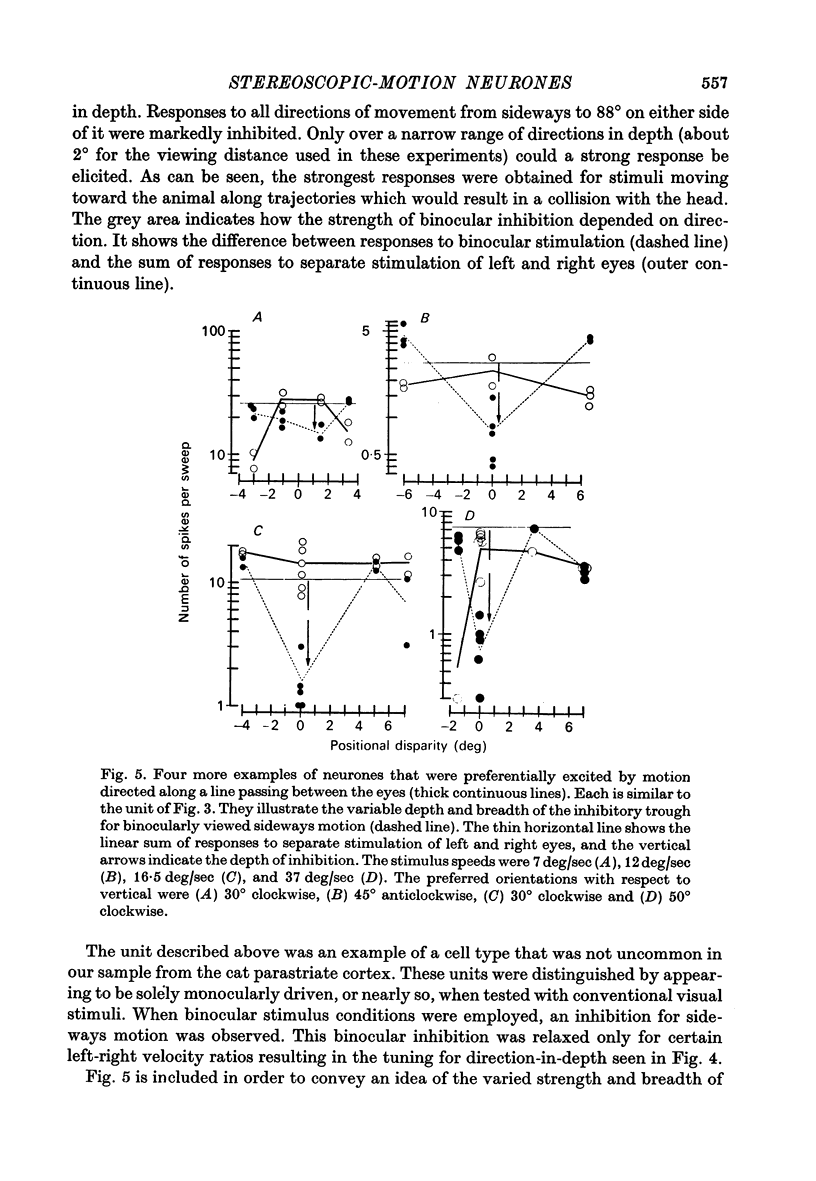
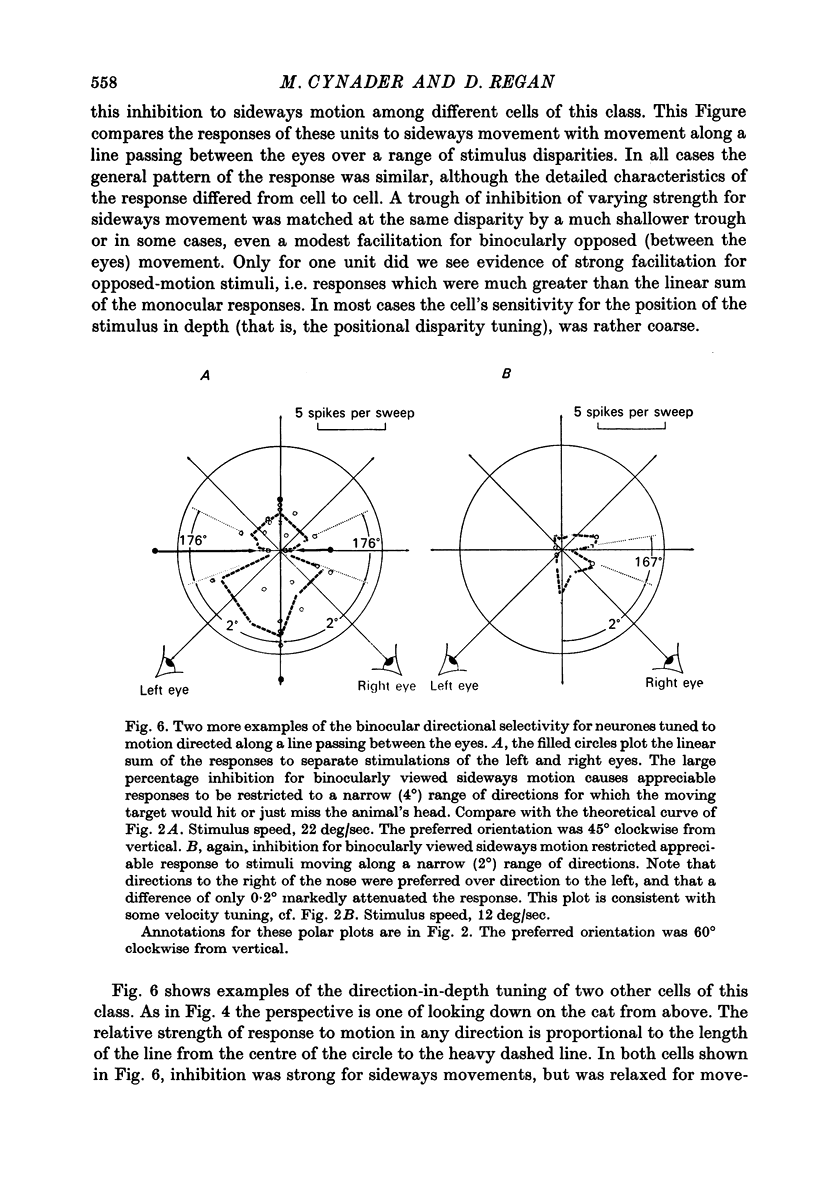
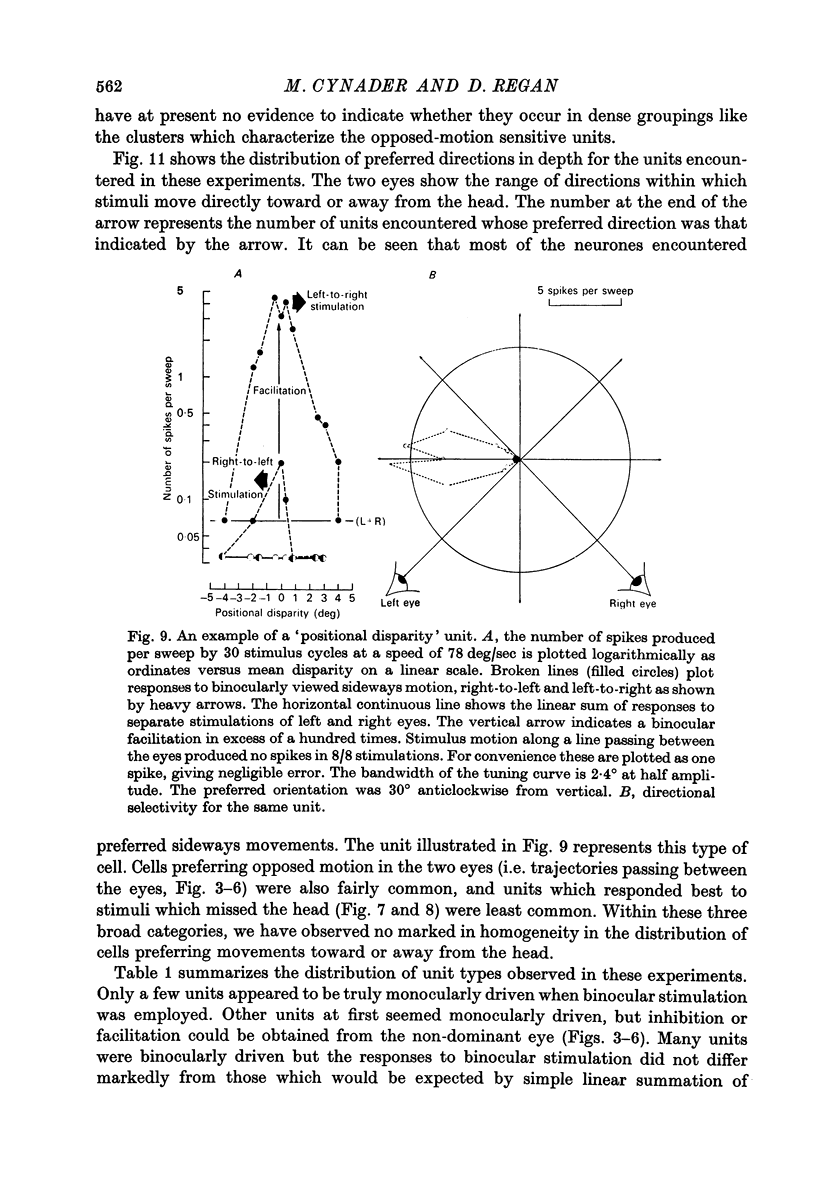
3. For one class of neurone (twenty cells), binocular stimulation inhibited firing for trajectories parallel to the frontoparallel plane over a large volume of space. Strong firing was produced by oppositely directed bar movements. Some of these neurones were especially narrowly tuned to the direction of movement in depth, responding only to a range of 2-3°, i.e. to moving bodies that would hit or only narrowly miss the cat. These cells emphasized the direction of movement at the expense of positional information.
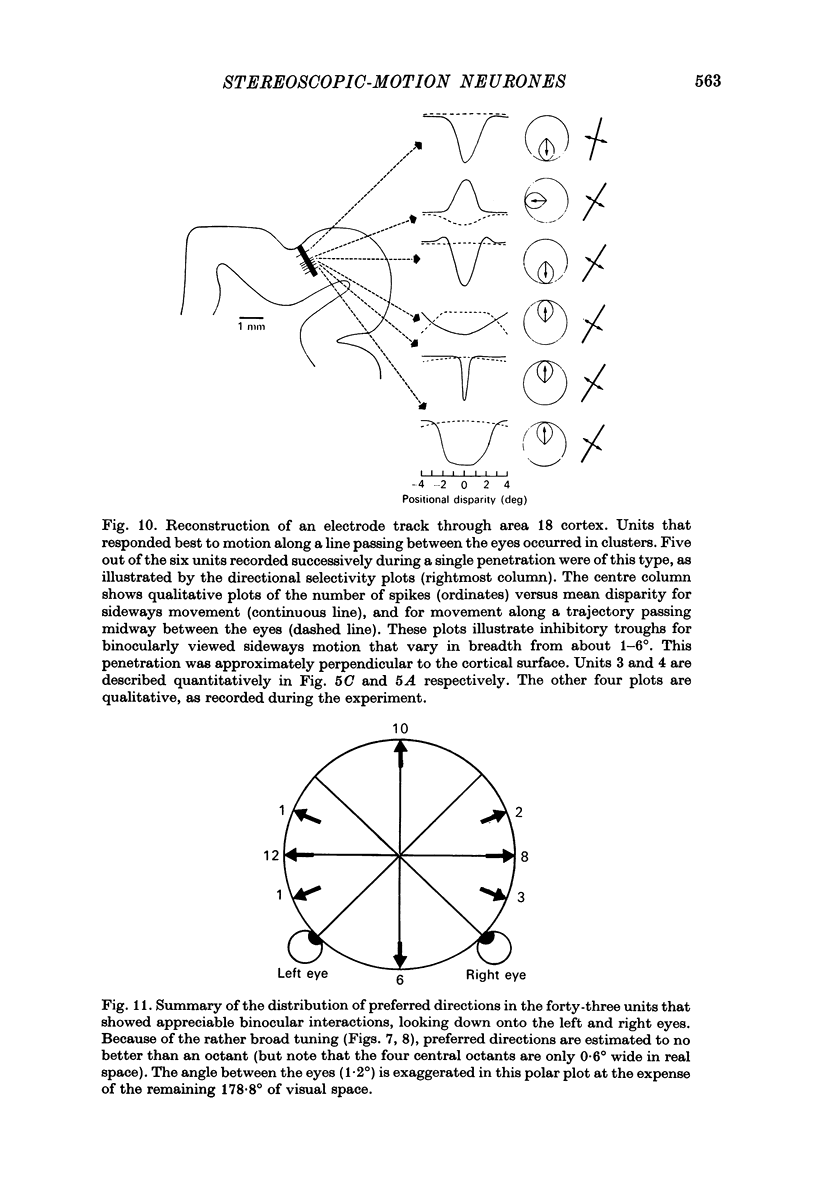
3. These units occurred in clusters. On the perpendicular penetrations in which they were found, they comprised a substantial majority of all cells encountered.
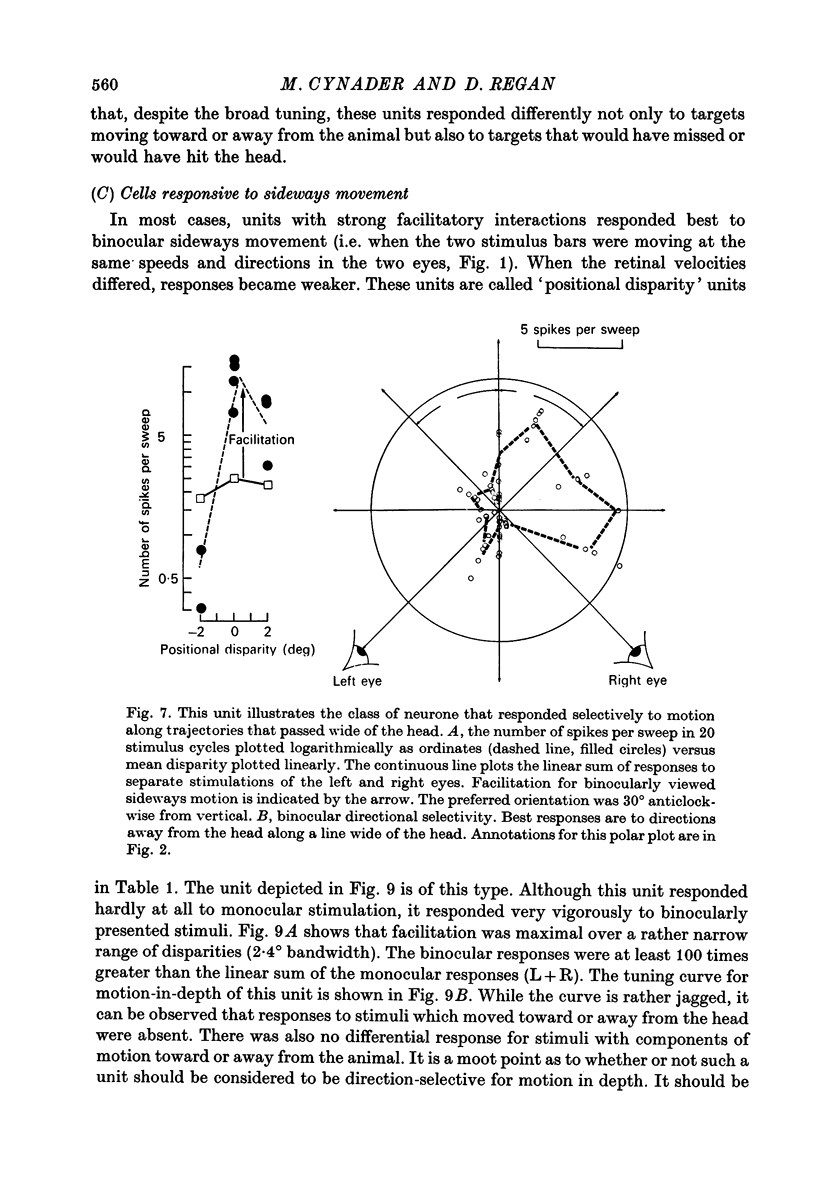
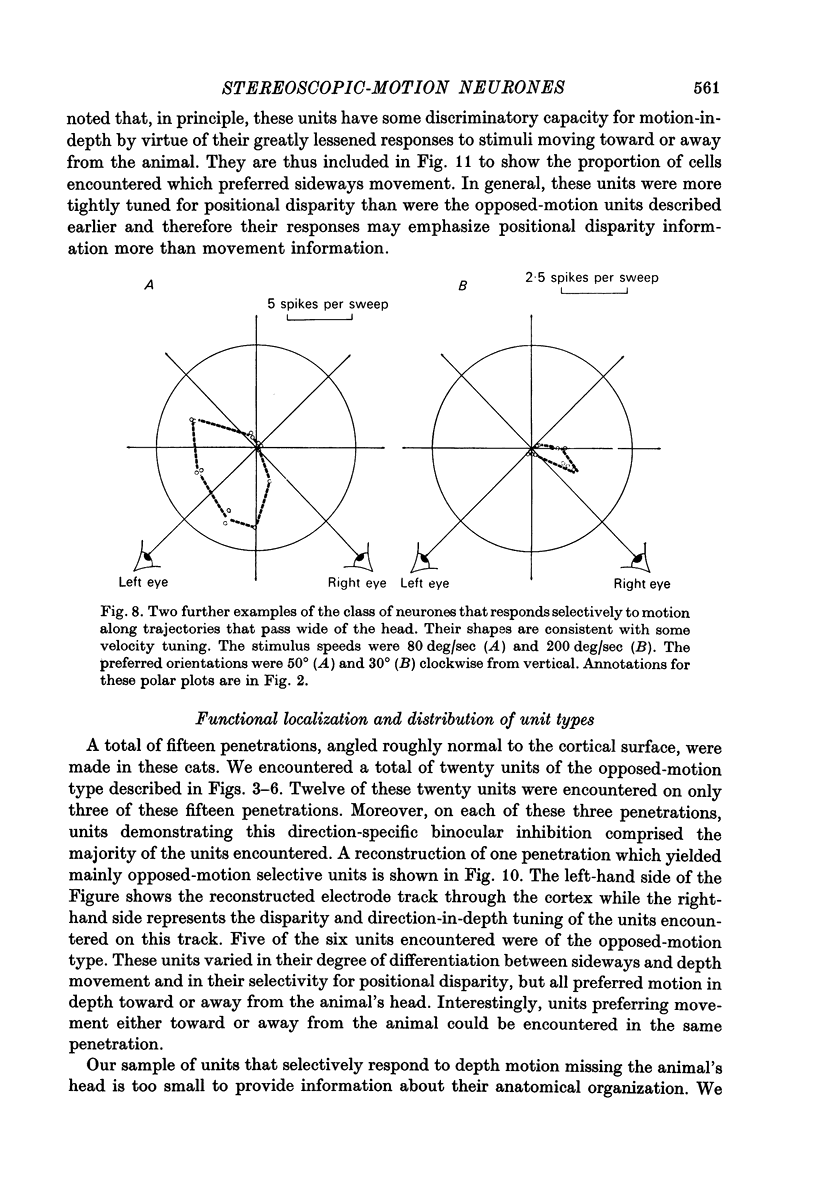
5. For a second class of neurone (nine cells), binocular facilitation produced selective responses to objects moving along trajectories that missed the head.
6. The two classes of neurone provide a basis for four proposed directionally tuned binocular motion detectors.
7. A third class of neurone (seventeen cells) was selectively sensitive to movements parallel to the frontoparallel plane. There was strong binocular facilitation when the bars moved at the same speeds in the same directions: oppositely directed movements might be more than 100 times less effective. These neurones may signal positional disparity.
8. These three classes of neurone cut across established categories. Only when both eyes were stimulated simultaneously with targets moving in different speeds and directions was it possible to demonstrate the binocular interactions described here.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B., Blakemore C., Pettigrew J. D. The neural mechanism of binocular depth discrimination. J Physiol. 1967 Nov;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman N., Blakemore C., Cynader M. Binocular interaction in the cat's superior colliculus. J Physiol. 1975 Apr;246(3):595–615. doi: 10.1113/jphysiol.1975.sp010906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley K. I., Regan D. Evidence for the existence of neural mechanisms selectively sensitive to the direction of movement in space. J Physiol. 1973 Nov;235(1):17–29. doi: 10.1113/jphysiol.1973.sp010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley K. I., Regan D. Selective adaptation in stereoscopic depth perception. J Physiol. 1973 Jul;232(1):40P–41P. [PubMed] [Google Scholar]
- Beverley K. I., Regan D. The relation between discrimination and sensitivity in the perception of motion in depth. J Physiol. 1975 Jul;249(2):387–398. doi: 10.1113/jphysiol.1975.sp011021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Henry G. H., Smith C. J. Binocular interaction fields of single units in the cat striate cortex. J Physiol. 1971 Jul;216(1):39–68. doi: 10.1113/jphysiol.1971.sp009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M., Berman N., Hein A. Recovery of function in cat visual cortex following prolonged deprivation. Exp Brain Res. 1976 May 28;25(2):139–156. doi: 10.1007/BF00234899. [DOI] [PubMed] [Google Scholar]
- Cynader M., Berman N. Receptive-field organization of monkey superior colliculus. J Neurophysiol. 1972 Mar;35(2):187–201. doi: 10.1152/jn.1972.35.2.187. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Stereoscopic vision in macaque monkey. Cells sensitive to binocular depth in area 18 of the macaque monkey cortex. Nature. 1970 Jan 3;225(5227):41–42. doi: 10.1038/225041a0. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B. Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol. 1957 Jul;20(4):408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D. Binocular neurons which signal change of disparity in area 18 of cat visual cortex. Nat New Biol. 1973 Jan 24;241(108):123–124. doi: 10.1038/newbio241123a0. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Binocular interaction on single units in cat striate cortex: simultaneous stimulation by single moving slit with receptive fields in correspondence. Exp Brain Res. 1968;6(4):391–410. doi: 10.1007/BF00233186. [DOI] [PubMed] [Google Scholar]
- Richards W., Regan D. A stereo field map with implications for disparity processing. Invest Ophthalmol. 1973 Dec;12(12):904–909. [PubMed] [Google Scholar]
- Rodieck R. W., Pettigrew J. D., Bishop P. O., Nikara T. Residual eye movements in receptive-field studies of paralyzed cats. Vision Res. 1967 Jan;7(1):107–110. doi: 10.1016/0042-6989(67)90031-4. [DOI] [PubMed] [Google Scholar]
- Stone J., Dreher B. Projection of X- and Y-cells of the cat's lateral geniculate nucleus to areas 17 and 18 of visual cortex. J Neurophysiol. 1973 May;36(3):551–567. doi: 10.1152/jn.1973.36.3.551. [DOI] [PubMed] [Google Scholar]
- Tretter F., Cynader M., Singer W. Cat parastriate cortex: a primary or secondary visual area. J Neurophysiol. 1975 Sep;38(5):1099–1113. doi: 10.1152/jn.1975.38.5.1099. [DOI] [PubMed] [Google Scholar]
- Wolbarsht M. L., Macnichol E. F., Jr, Wagner H. G. Glass Insulated Platinum Microelectrode. Science. 1960 Nov 4;132(3436):1309–1310. doi: 10.1126/science.132.3436.1309. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Cells responding to changing image size and disparity in the cortex of the rhesus monkey. J Physiol. 1974 Nov;242(3):827–841. doi: 10.1113/jphysiol.1974.sp010736. [DOI] [PMC free article] [PubMed] [Google Scholar]


