Abstract
1. Intracellular injection, recording and current-passing methods were used to investigate the role of intracellular Ca in the modulation of electrical behaviour in the ciliate Paramecium caudatum.
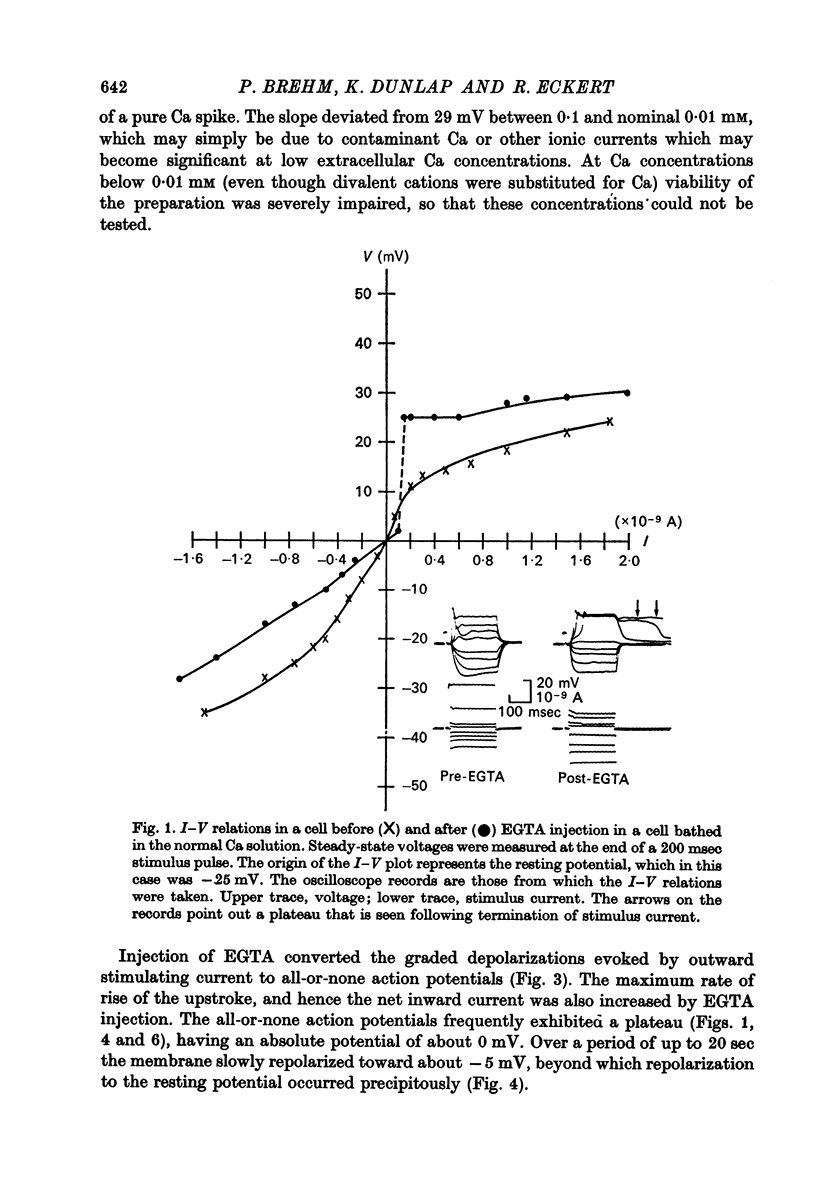
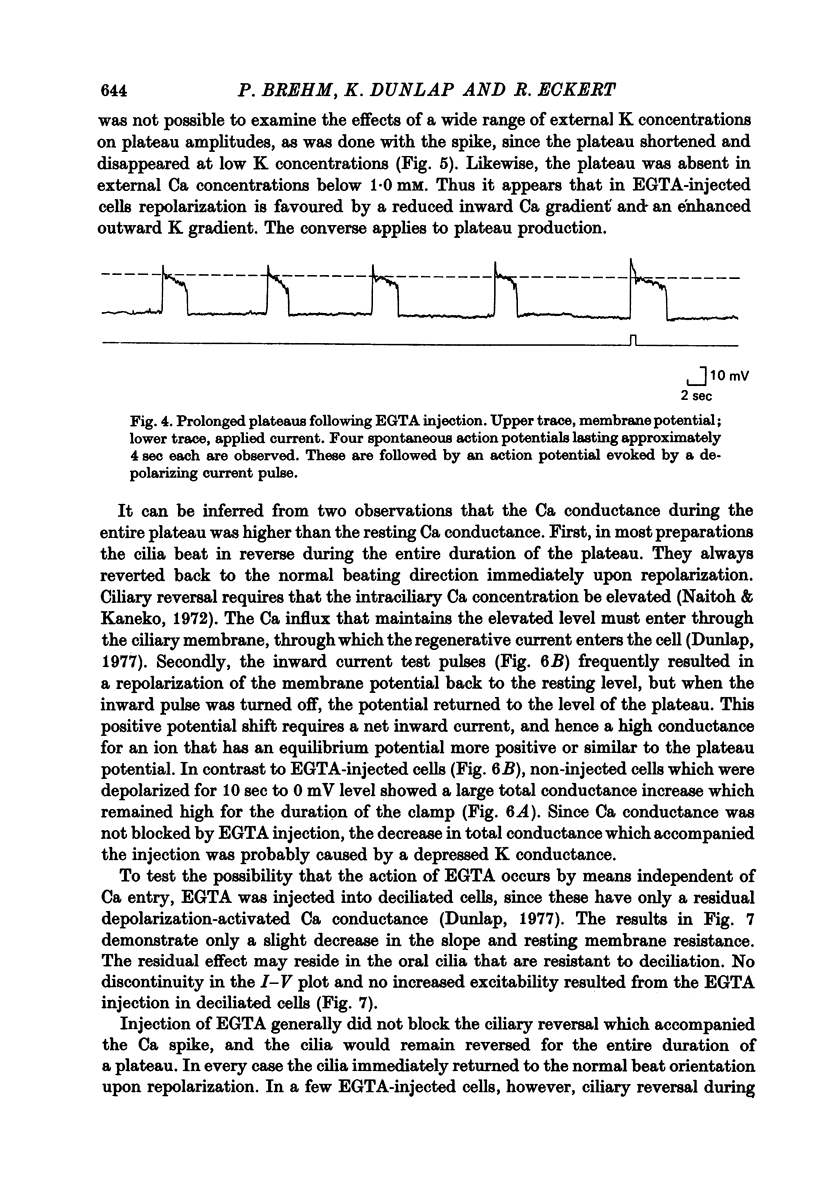
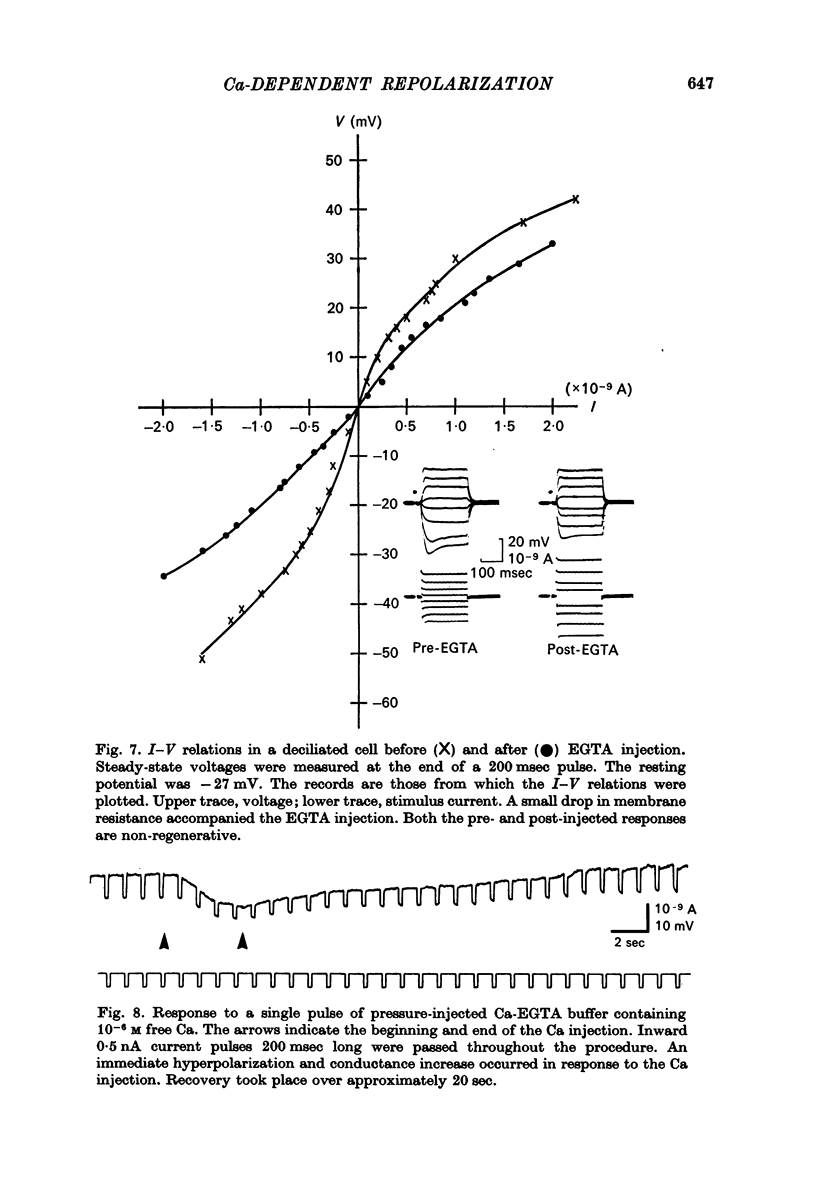
2. Injection of EGTA converted graded regenerative responses ascribed to Ca inward current to all-or-none action potentials. The EGTA injection also caused a discontinuity in the steady state I—V relations to outward current, but had little effect on hyperpolarizing current—voltage responses.
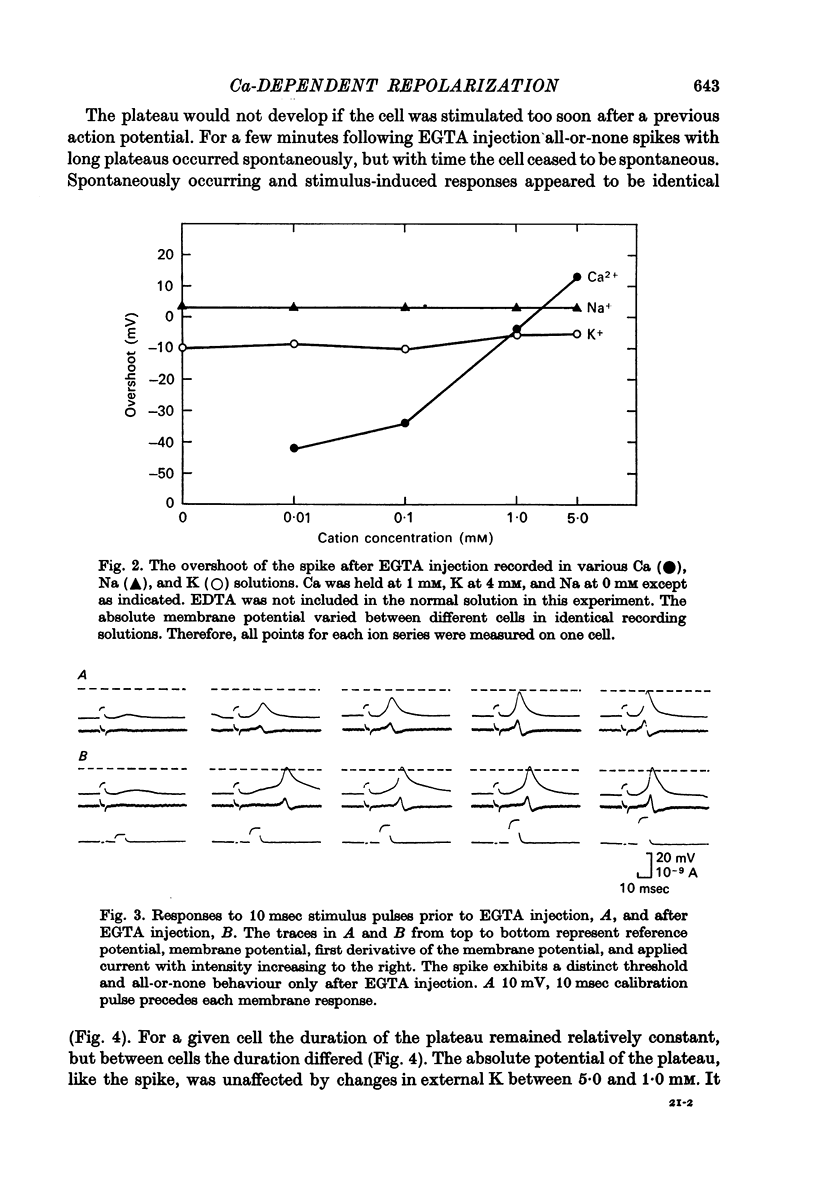
3. The overshoot of the all-or-none spike produced by the EGTA-injected cell followed an approximate 29 mV increase for a tenfold increase in external Ca concentration and was independent of changes in external K and Na concentrations.
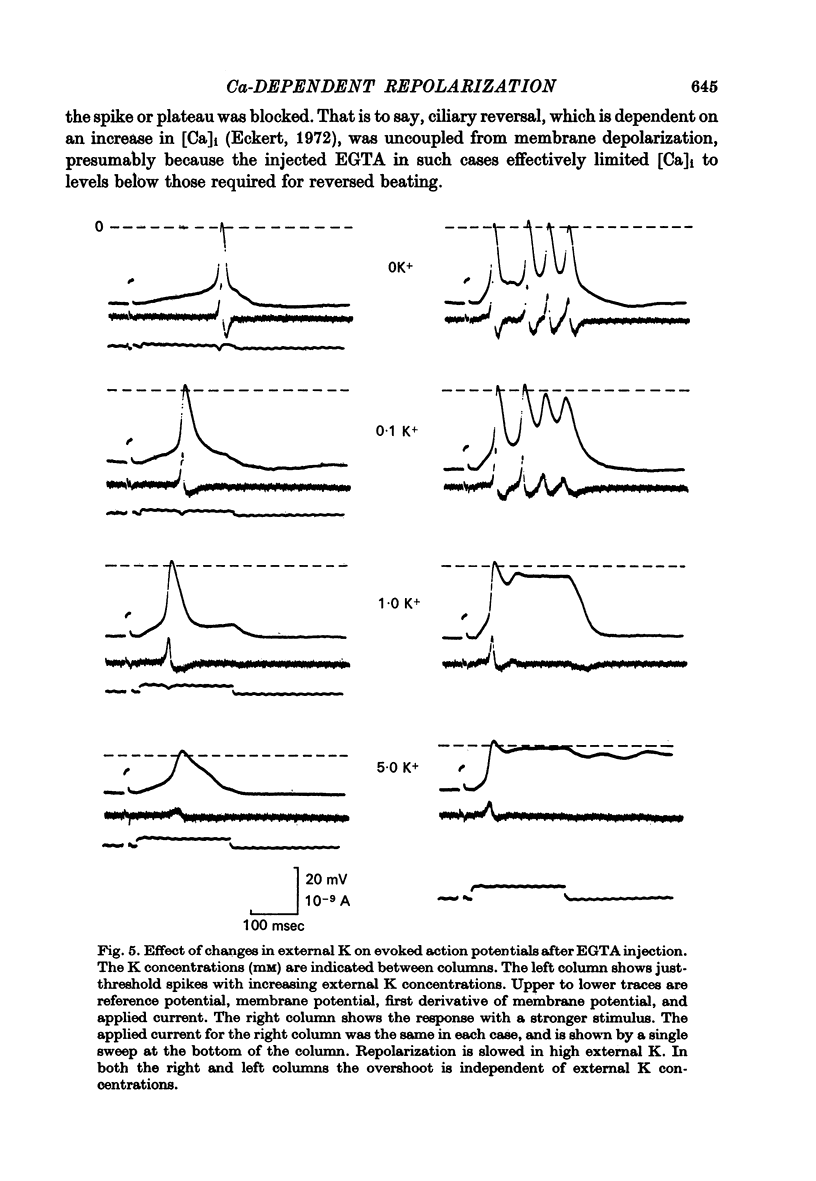
4. The EGTA-induced all-or-none action potential tended to produce plateaus that could last up to 20 sec. During the plateau the membrane slowly repolarized to a critical potential, upon which repolarization occurred precipitously.
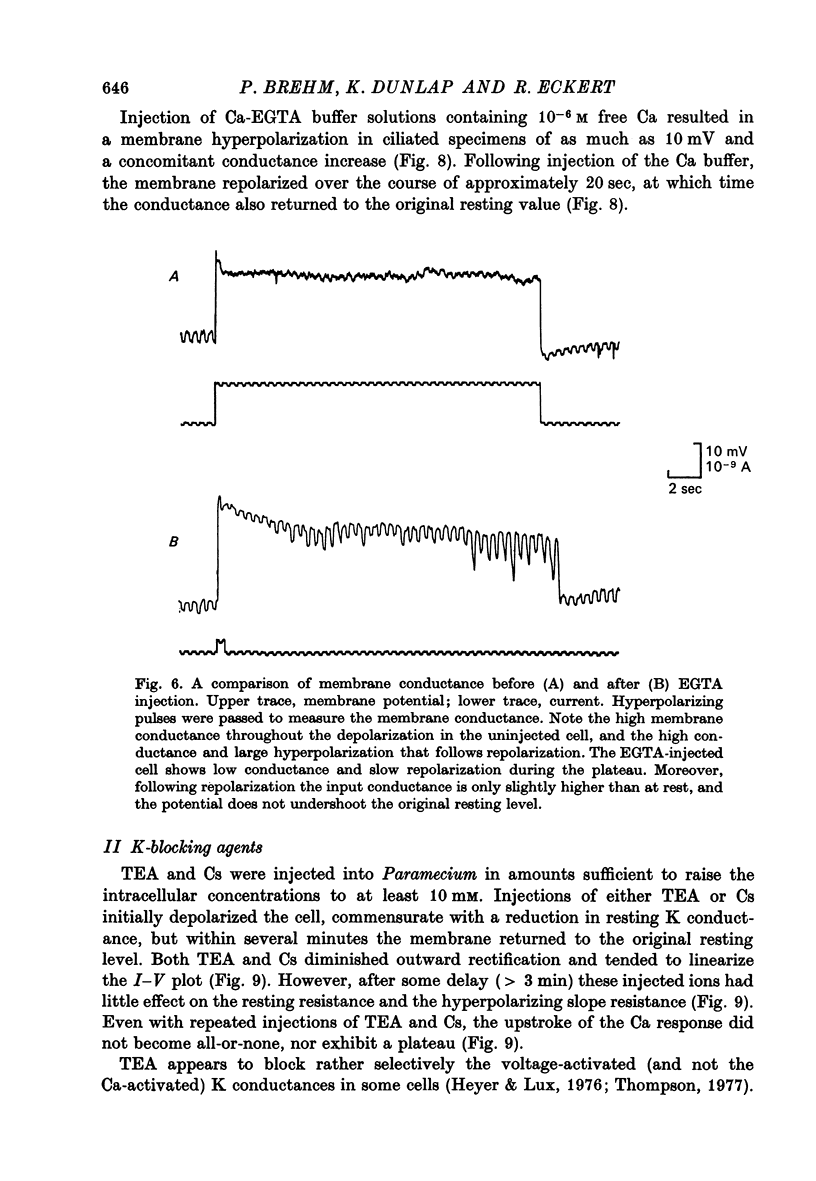
5. Injection of 10-6 M-free Ca2+ as a Ca-EGTA buffer hyperpolarized the membrane and decreased the potential shifts to inward current pulses. These responses are consistent with an increase in K conductance.
6. During EGTA plateaus reversed beating of the cilia indicated a rise in intracellular Ca, and thus an inability of the EGTA to complex the Ca as rapidly as it entered the cilia. Reversal of the motile apparatus thus appears to be activated at lower concentrations of intracellular Ca than are required to activate the inferred Ca-dependent K system.
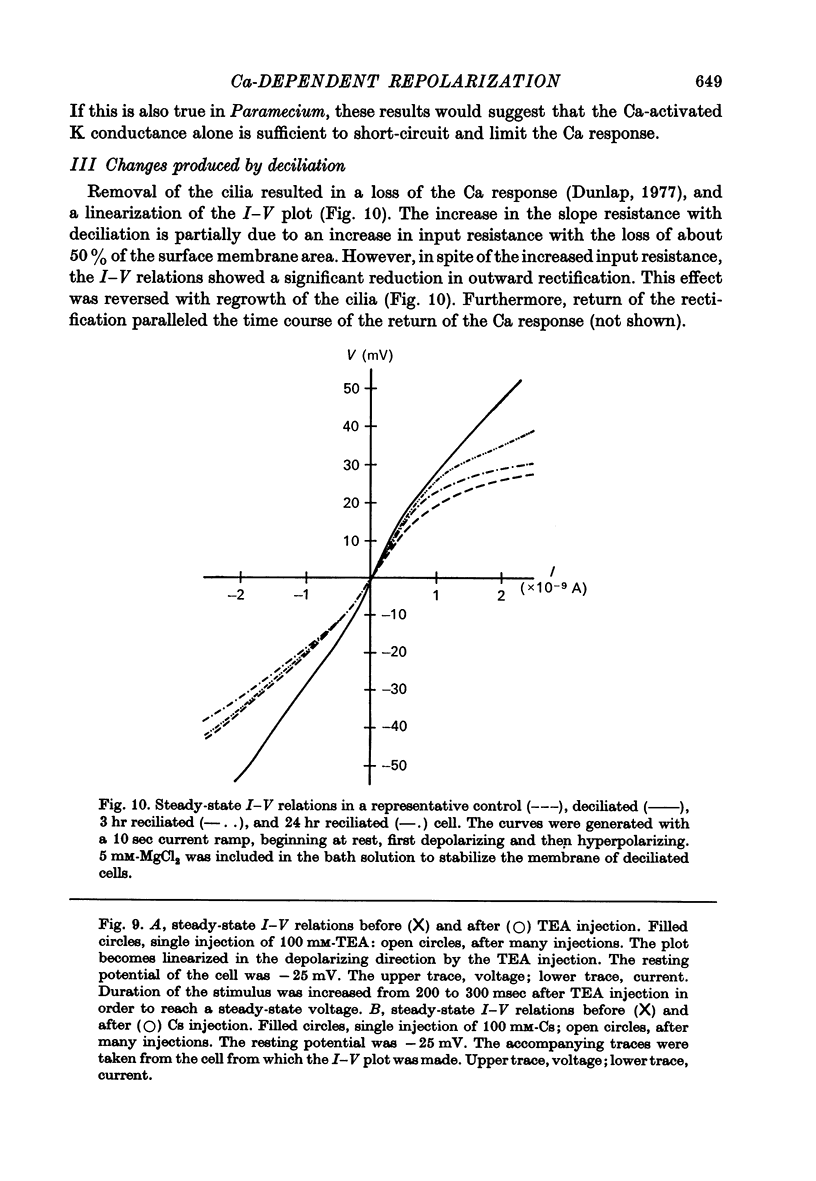
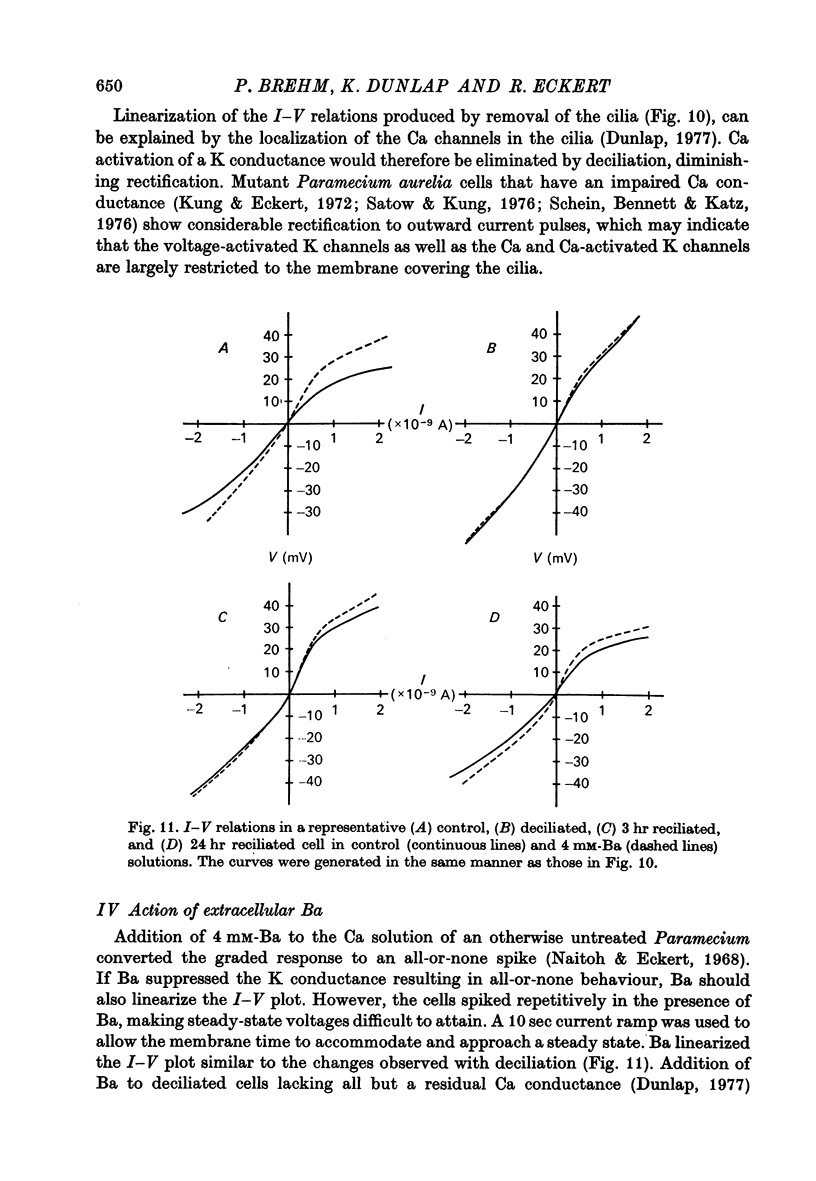
7. In uninjected cells removal of the cilia, which results in a loss of the voltage-activated Ca channels (Dunlap, 1977), or addition of extracellular Ba both tended to linearize the steady state I—V relations.
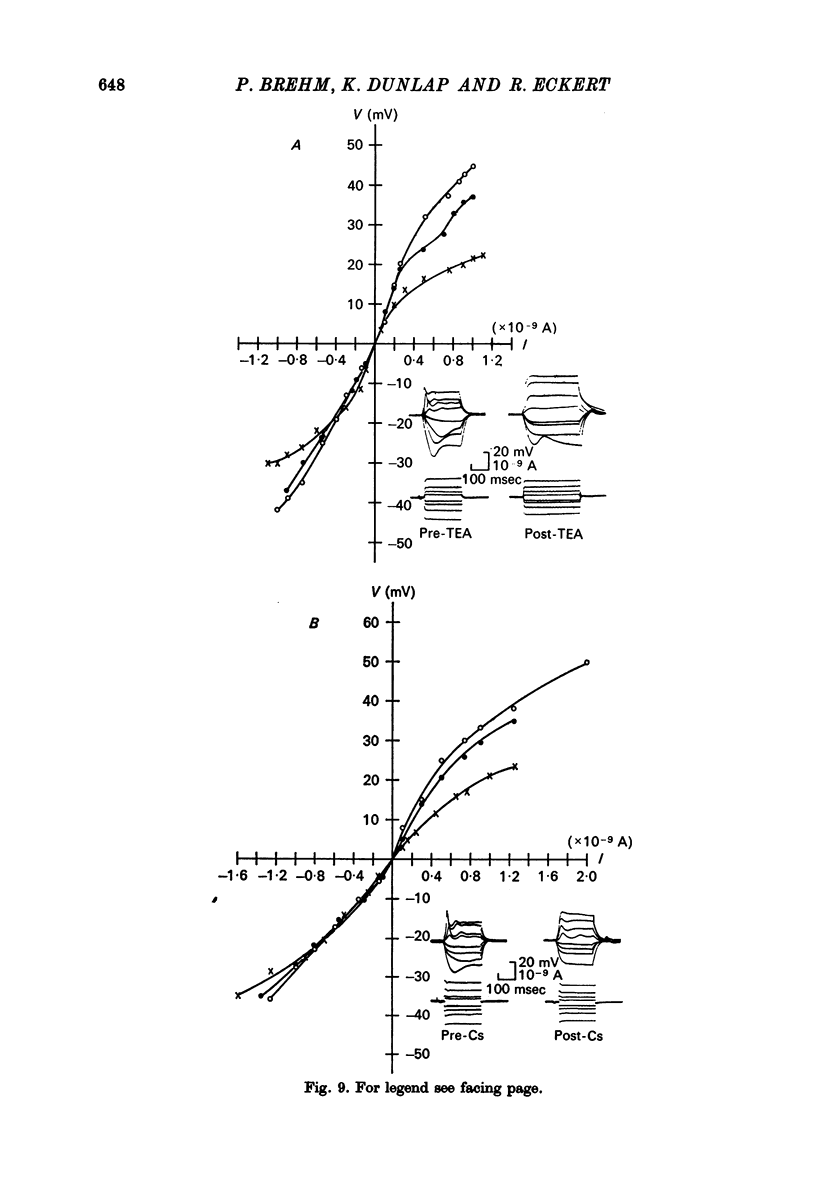
8. Injections of Cs and TEA tended to linearize the steady state I—V relations, but did not result in either a conversion to an all-or-none spike or a discontinuity in the depolarizing steady-state I—V relations.
9. It is concluded that in Paramecium a Ca-activated K conductance short-circuits the inward current of the regenerative Ca response, preventing all-or-none behaviour. The occurrence of plateau spikes following EGTA injection indicates that the Ca conductance inactivates very slowly in face of a maintained depolarization. Such slow Ca-inactivation is consistent with the slow relaxation of Ca-dependent ciliary reversal that occurs during maintained depolarization.
10. The possibility is discussed that injection of EGTA may also enhance the Ca conductance.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clusin W., Spray D. C., Bennett M. V. Activation of a voltage-insensitive conductance by inward calcium current. Nature. 1975 Jul 31;256(5516):425–427. doi: 10.1038/256425a0. [DOI] [PubMed] [Google Scholar]
- Dunlap K. Localization of calcium channels in Paramecium caudatum. J Physiol. 1977 Sep;271(1):119–133. doi: 10.1113/jphysiol.1977.sp011993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R. Bioelectric control of ciliary activity. Science. 1972 May 5;176(4034):473–481. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- Eckert R. Genes, channels and membrane currents in Paramecium. Nature. 1977 Jul 14;268(5616):104–105. doi: 10.1038/268104a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Fukuda J., Eaton D. C. Membrane currents carried by Ca, Sr, and Ba in barnacle muscle fiber during voltage clamp. J Gen Physiol. 1974 May;63(5):564–578. doi: 10.1085/jgp.63.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Control of the delayed outward potassium currents in bursting pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):349–382. doi: 10.1113/jphysiol.1976.sp011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnberg G. Is potassium conductance of cardiac Purkinje fibres controlled by (Ca2+)? Nature. 1975 Jan 24;253(5489):273–274. doi: 10.1038/253273a0. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Lisiewicz A. Injections of calcium ions into spinal motoneurones. J Physiol. 1972 Sep;225(2):363–390. doi: 10.1113/jphysiol.1972.sp009945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung C., Eckert R. Genetic modification of electric properties in an excitable membrane (paramecium-calcium conductance-electrophysiological measurements-membrane mutant). Proc Natl Acad Sci U S A. 1972 Jan;69(1):93–97. doi: 10.1073/pnas.69.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Kaneko H. Reactivated triton-extracted models o paramecium: modification of ciliary movement by calcium ions. Science. 1972 May 5;176(4034):523–524. doi: 10.1126/science.176.4034.523. [DOI] [PubMed] [Google Scholar]
- Naitoh Y., Eckert R., Friedman K. A regenerative calcium response in Paramecium. J Exp Biol. 1972 Jun;56(3):667–681. doi: 10.1242/jeb.56.3.667. [DOI] [PubMed] [Google Scholar]
- Oertel D., Schein S. J., Kung C. Separation of membrane currents using a Paramecium mutant. Nature. 1977 Jul 14;268(5616):120–124. doi: 10.1038/268120a0. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Satow Y., Kung C. Mutants with reduced Ca activation in Paramecium aurelia. J Membr Biol. 1976 Aug 26;28(2-3):277–294. doi: 10.1007/BF01869701. [DOI] [PubMed] [Google Scholar]
- Schein S. J., Bennett M. V., Katz G. M. Altered calcium conductance in pawns, behavioural mutants of Paramecium aurelia. J Exp Biol. 1976 Dec;65(3):699–724. doi: 10.1242/jeb.65.3.699. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R. Control of membrane permeability to potassium in red blood cells. Nature. 1968 Aug 10;219(5154):610–610. doi: 10.1038/219610a0. [DOI] [PubMed] [Google Scholar]


