Abstract
1. The time course of the uptake of [14C]urea by the lateral ventricular choroid plexus of the adult rat in vivo was analysed to delineate further the permeability characteristics of the epithelial membrane of this secretory tissue.
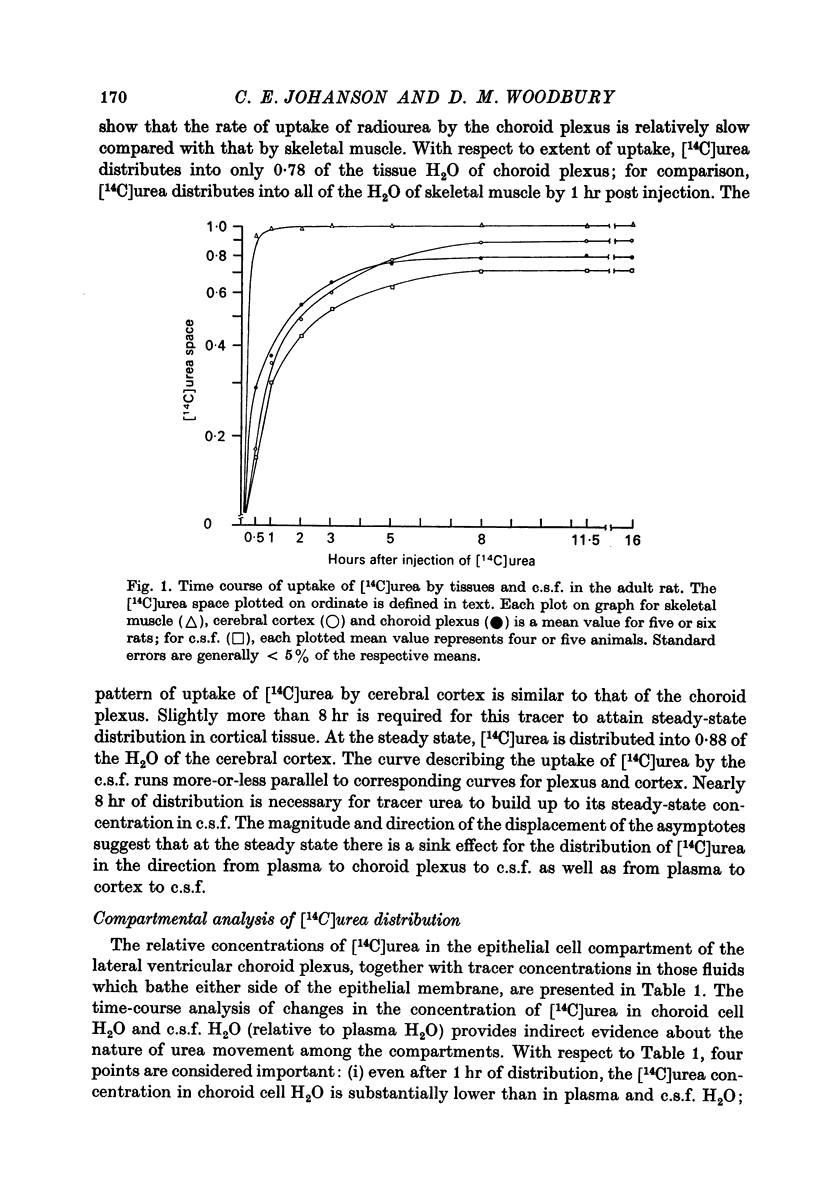
2. Eight hours after I.P. injection, [14C]urea attained a steady-state distribution in 78% of the tissue water of lateral ventricular choroid plexus; similarly, approximately 8 hr was required for radiourea to reach a steady-state concentration in both the cerebral cortex and cerebrospinal fluid (c.s.f.).
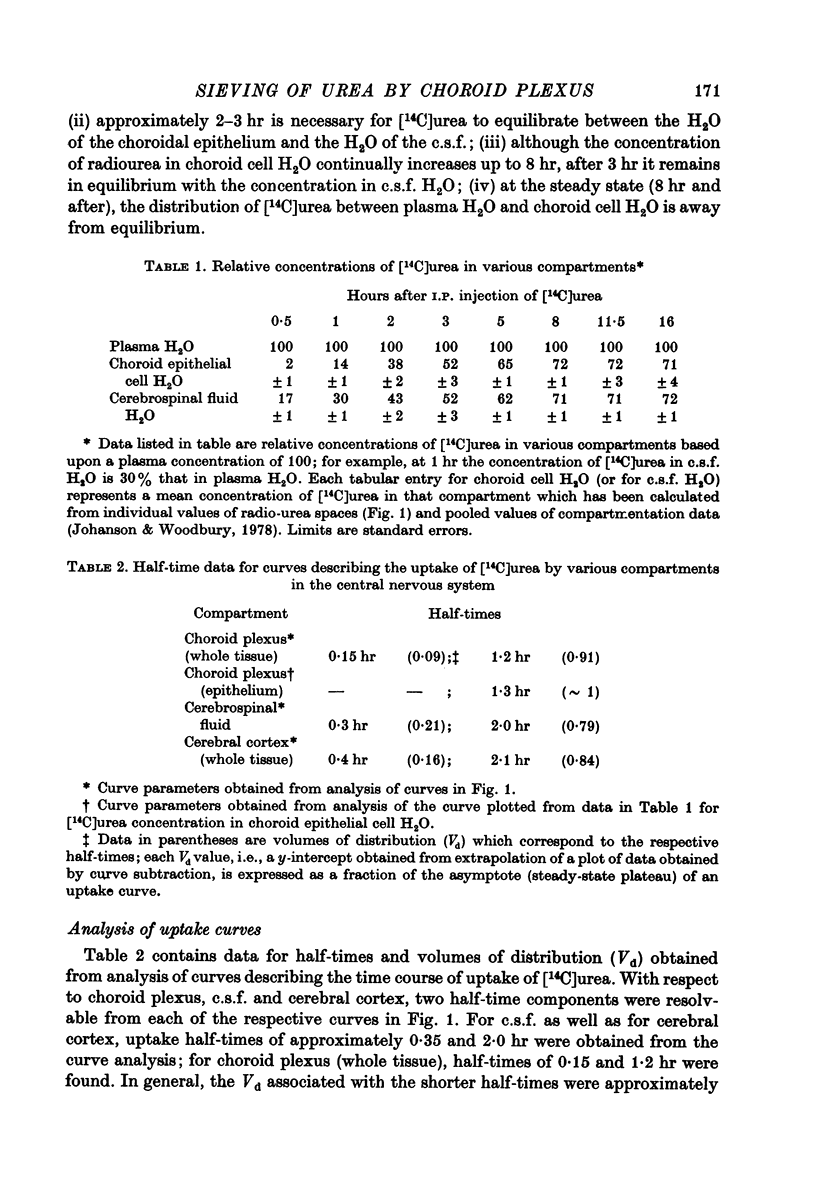
3. Results obtained for compartment analysis were used to calculate the concentration of [14C]urea in the epithelium of the lateral ventricular plexus during the approach to and at steady-state distribution. Even after 1 hr of distribution, the [14C]urea concentration in choroid cell water was less than 15% of that in plasma water.
4. Although the concentration of radiourea in choroid cell water continually increased after 3 hr, it remained in equilibrium with the concentration of [14C]urea in c.s.f. water. At the steady state (i.e., 8 hr), the distribution of [14C]urea between the water of plasma and that of the choroidal epithelium was considerably away from equilibrium (i.e., by 25-30%).
5. An analysis of the concentration gradients for [14C]urea across both the apical (c.s.f.-facing) and basolateral (plasma-facing) membranes of the epithelium of the lateral ventricular plexus suggests that the movement of urea is hindered to a greater extent by the basolateral membrane than by the apical membrane.
6. Only a single half-time component (1·3 hr) can be resolved from analysis of the curve describing the time course of uptake of radiourea by the choroid epithelial cell compartment.
7. The concentration gradient data suggest that urea penetrates from blood to c.s.f. via the choroid plexus by a transcellular pathway; however, it is not possible to rule out a paracellular pathway for urea movement.
8. At the steady state, radiourea distributes into 88% of the water of cerebral cortex. This observation, together with the finding of a steady-state concentration gradient for [14C]urea from cortical tissue to c.s.f., constitutes evidence that urea movement is hindered at the blood-brain barrier as well as at the blood—c.s.f. barrier.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADBURY M. W., COXON R. V. The penetration of urea into the central nervous system at high blood levels. J Physiol. 1962 Oct;163:423–435. doi: 10.1113/jphysiol.1962.sp006987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADBURY M. W., DAVSON H. THE TRANSPORT OF UREA, CREATININE AND CERTAIN MONOSACCHARIDES BETWEEN BLOOD AND FLUID PERFUSING THE CEREBRAL VENTRICULAR SYSTEM OF RABBITS. J Physiol. 1964 Jan;170:195–211. doi: 10.1113/jphysiol.1964.sp007323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADBURY M. W., STUBBS J., HUGHES I. E., PARKER P. THE DISTRIBUTION OF POTASSIUM, SODIUM, CHLORIDE AND UREA BETWEEN LUMBAR CEREBROSPINAL FLUID AND BLOOD SERUM IN HUMAN SUBJECTS. Clin Sci. 1963 Aug;25:97–105. [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserr H. F. Relationship between cerebrospinal fluid and interstitial fluid of brain. Fed Proc. 1974 Sep;33(9):2075–2078. [PubMed] [Google Scholar]
- DAVSON H., KLEEMAN C. R., LEVIN E. Quantitative studies of the passage of different substances out of the cerebrospinal fluid. J Physiol. 1962 Apr;161:126–142. doi: 10.1113/jphysiol.1962.sp006877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER R. G., COPENHAVER J. H., Jr, MALINE D. S. Effect of urea on enzyme activity of the choroid plexus. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:797–799. doi: 10.3181/00379727-101-25099. [DOI] [PubMed] [Google Scholar]
- Fenstermacher J. D., Patlak C. S., Blasberg R. G. Transport of material between brain extracellular fluid, brain cells and blood. Fed Proc. 1974 Sep;33(9):2070–2074. [PubMed] [Google Scholar]
- GILBOE D., JAVID M., FRECHETTE P. The fate and distribution of hypertonic urea solutions: a preliminary report. Surg Forum. 1960;11:390–391. [PubMed] [Google Scholar]
- Gabriel R. Letter: Variations in plasma urea and creatinine. Br Med J. 1975 Feb 1;1(5952):272–272. doi: 10.1136/bmj.1.5952.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson C. E., Reed D. J., Woodbury D. M. Active transport of sodium and potassium by the choroid plexus of the rat. J Physiol. 1974 Sep;241(2):359–372. doi: 10.1113/jphysiol.1974.sp010660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson C. E., Reed D. J., Woodbury D. M. Developmental studies of the compartmentalization of water and electrolytes in the choroid plexus of the neonatal rat brain. Brain Res. 1976 Oct 29;116(1):35–48. doi: 10.1016/0006-8993(76)90247-x. [DOI] [PubMed] [Google Scholar]
- Johanson C. E., Woodbury D. M. Penetration of 14C-antipyrine and 14C-barbital into the choroid plexus and cerebrospinal fluid of the rat in vivo. Exp Brain Res. 1977 Oct 24;30(1):65–74. doi: 10.1007/BF00237859. [DOI] [PubMed] [Google Scholar]
- KLEEMAN C. R., DAVSON H., LEVIN E. Urea transport in the central nervous system. Am J Physiol. 1962 Oct;203:739–747. doi: 10.1152/ajplegacy.1962.203.4.739. [DOI] [PubMed] [Google Scholar]
- MAXWELL D. S., PEASE D. C. The electron microscopy of the choroid plexus. J Biophys Biochem Cytol. 1956 Jul 25;2(4):467–474. doi: 10.1083/jcb.2.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollay M., Kaplan R. J. Diffusion of non-electrolytes in brain tissue. Brain Res. 1970 Feb 3;17(3):407–416. doi: 10.1016/0006-8993(70)90249-0. [DOI] [PubMed] [Google Scholar]
- REED D. J., WOODBURY D. M. Effect of hypertonic urea on cerebrospinal fluid pressure and brain volume. J Physiol. 1962 Nov;164:252–264. doi: 10.1113/jphysiol.1962.sp007018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L. E. Organ distribution of exogenous 14 C-urea in elasmobranchs, with special regard to the nervous system. Comp Biochem Physiol A Comp Physiol. 1971 Sep 1;40(1):145–154. doi: 10.1016/0300-9629(71)90157-5. [DOI] [PubMed] [Google Scholar]
- SCHOOLAR J. C., BARLOW C. F., ROTH L. J. The penetration of carbon-14 urea into cerebrospinal fluid and various areas of the cat brain. J Neuropathol Exp Neurol. 1960 Apr;19:216–227. doi: 10.1097/00005072-196004000-00003. [DOI] [PubMed] [Google Scholar]
- WELCH K. SECRETION OF CEREBROSPINAL FLUID BY CHOROID PLEXUS OF THE RABBIT. Am J Physiol. 1963 Sep;205:617–624. doi: 10.1152/ajplegacy.1963.205.3.617. [DOI] [PubMed] [Google Scholar]
- Welch K., Sadler K. Permeability of the choroid plexus of the rabbit to several solutes. Am J Physiol. 1966 Mar;210(3):652–660. doi: 10.1152/ajplegacy.1966.210.3.652. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Pietras R. J. Routes of nonelectrolyte permeation across epithelial membranes. J Membr Biol. 1974 Jul 12;17(3):293–312. doi: 10.1007/BF01870189. [DOI] [PubMed] [Google Scholar]


