Abstract
1. The effects of different external ionic conditions and of metabolic inhibitors on the membrane potential of hyperpolarizing photoreceptors in the retina of the scallop Pecten irradians were examined in the presence and absence of light.
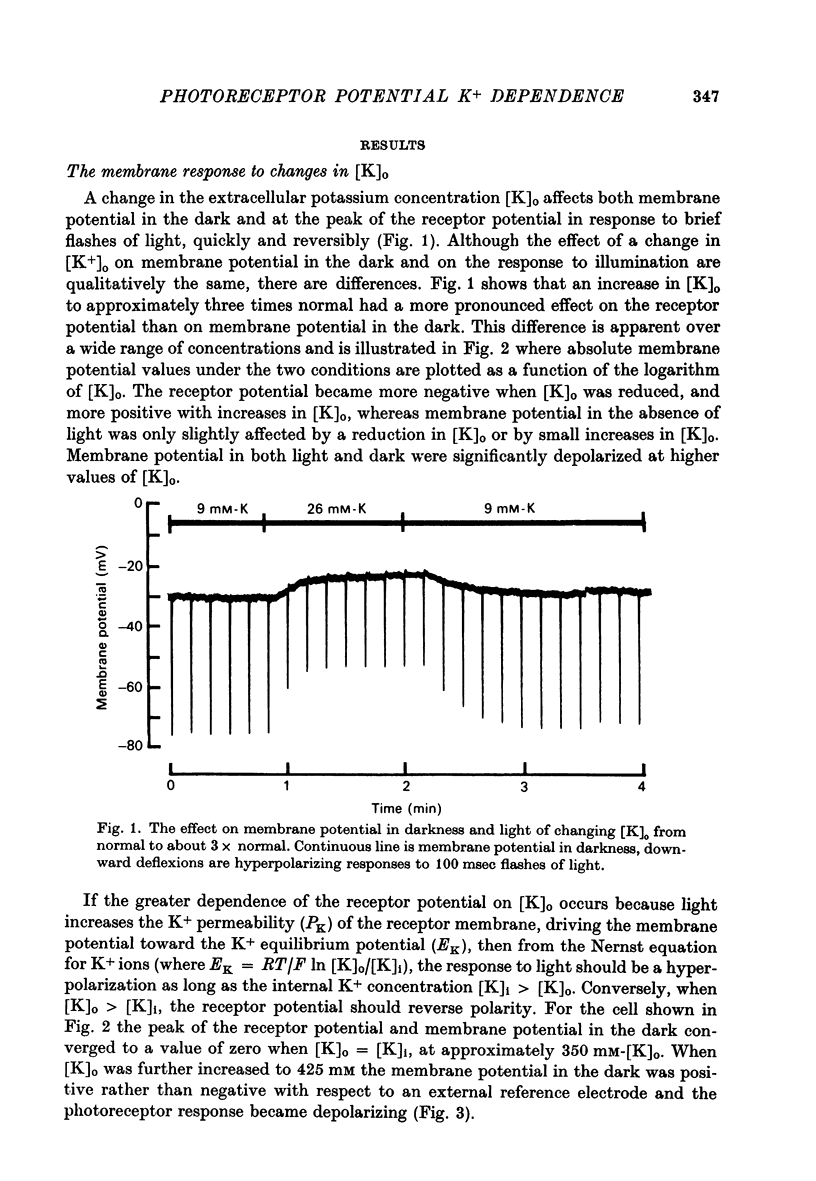
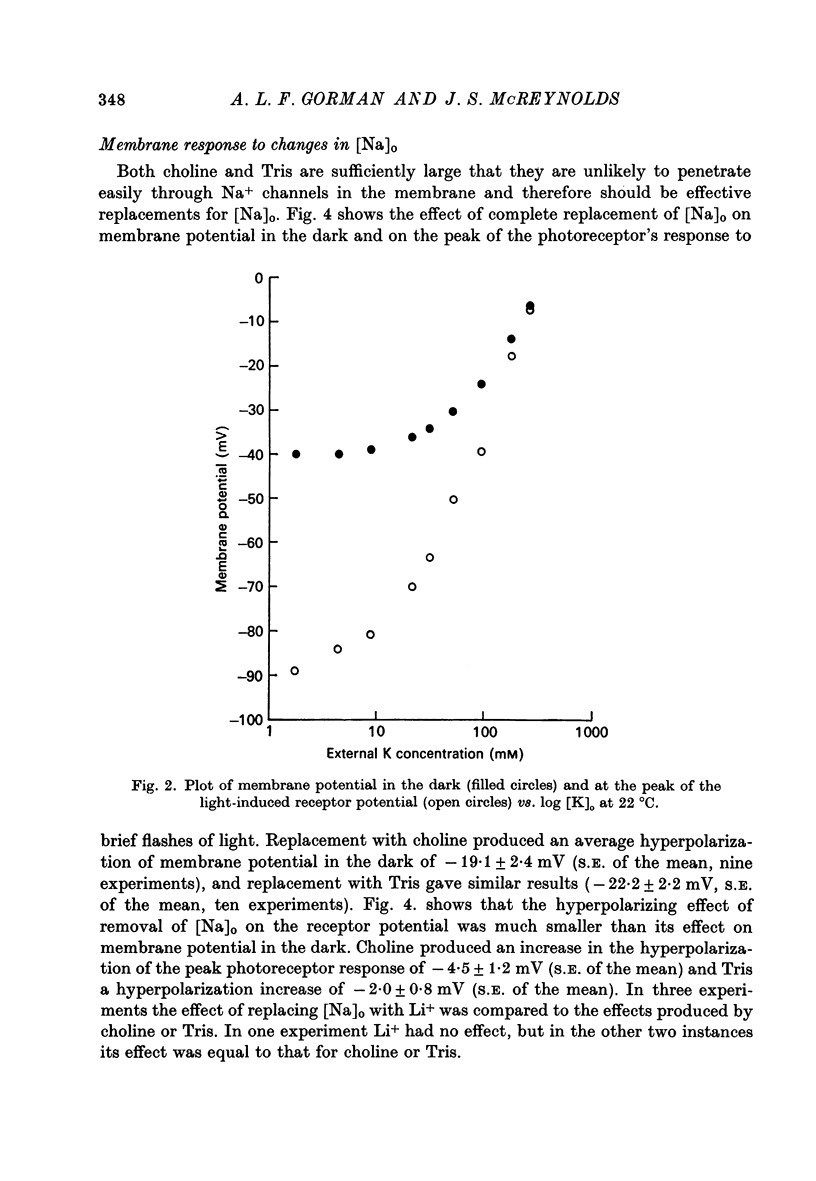
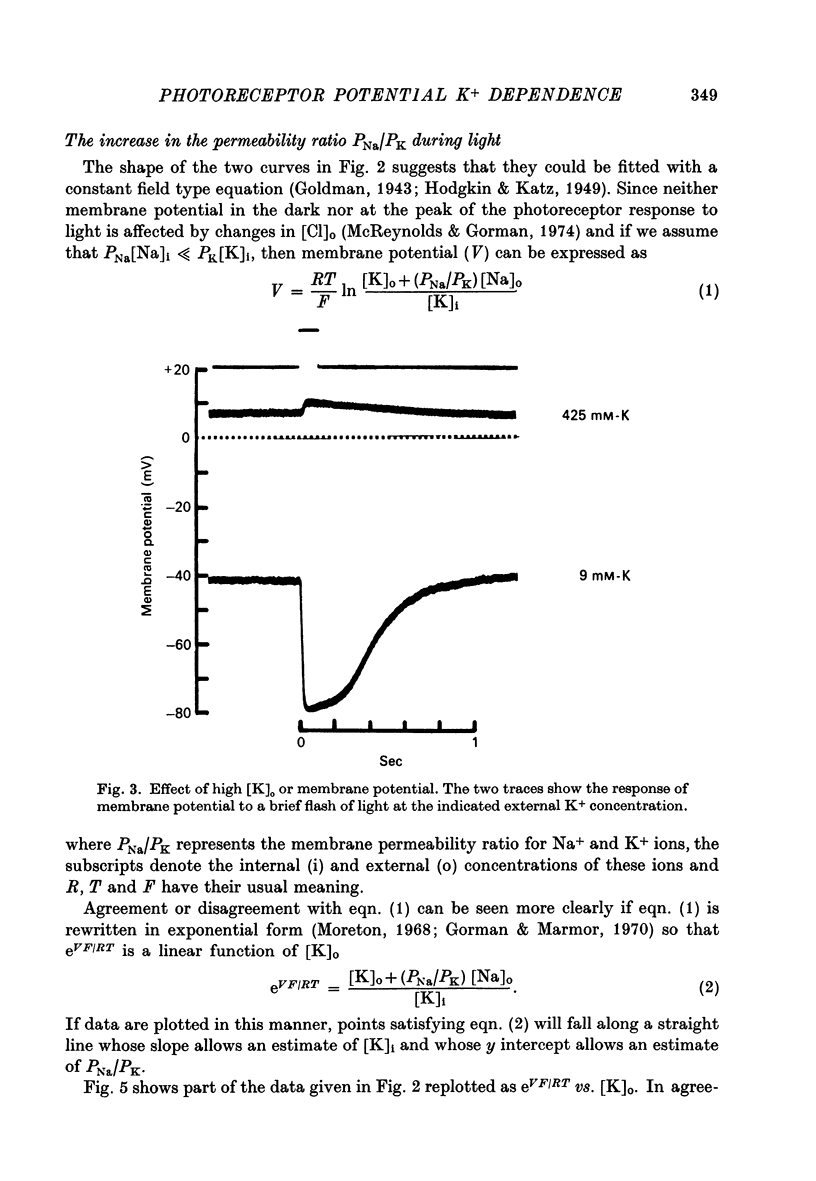
2. Changes in extracellular K+ have a greater effect on membrane potential in the light than in darkness. The receptor potential is increased in amplitude when [K]o is reduced and decreased when [K]o is elevated. It is hyperpolarizing when [K]o is less than the estimated value for [K]i and depolarizing when this condition is reversed.
3. The complete replacement of [Na]o causes a significant hyperpolarization of membrane potential in darkness, whereas it has a much smaller hyperpolarizing effect on the peak of the receptor potential.
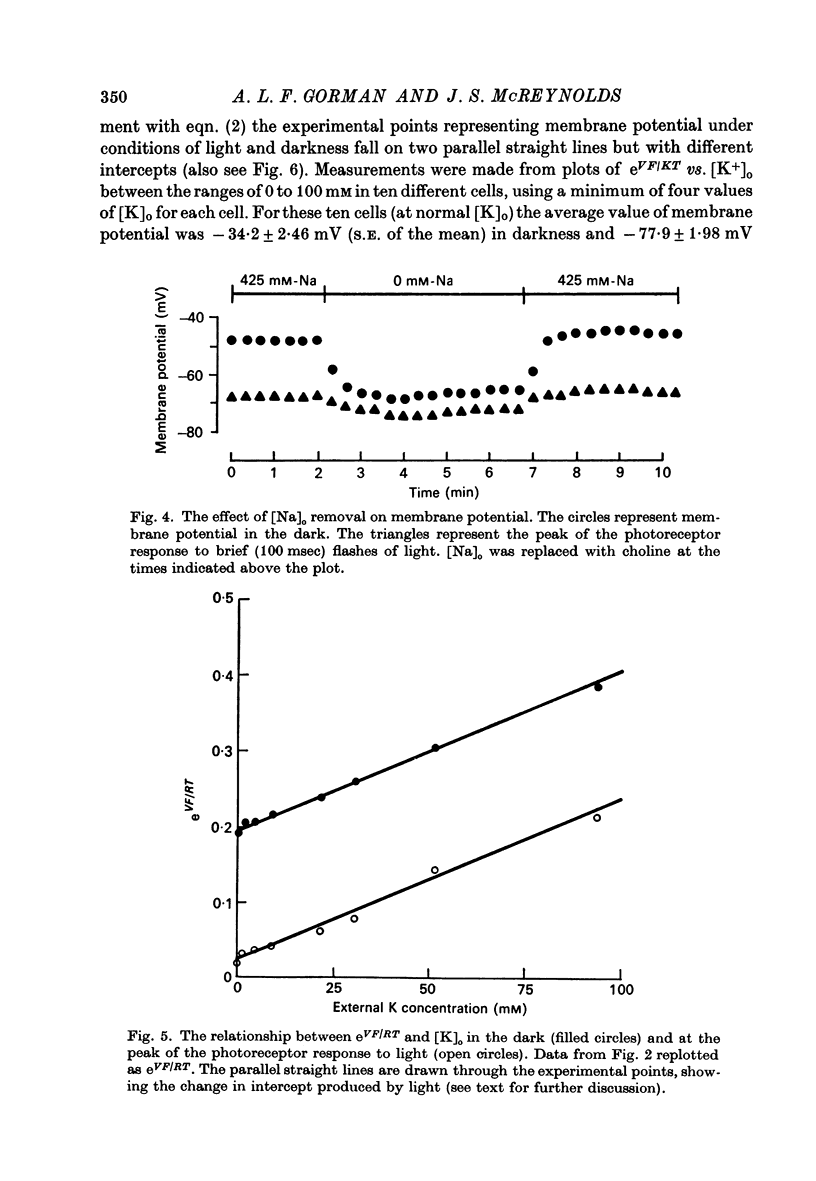
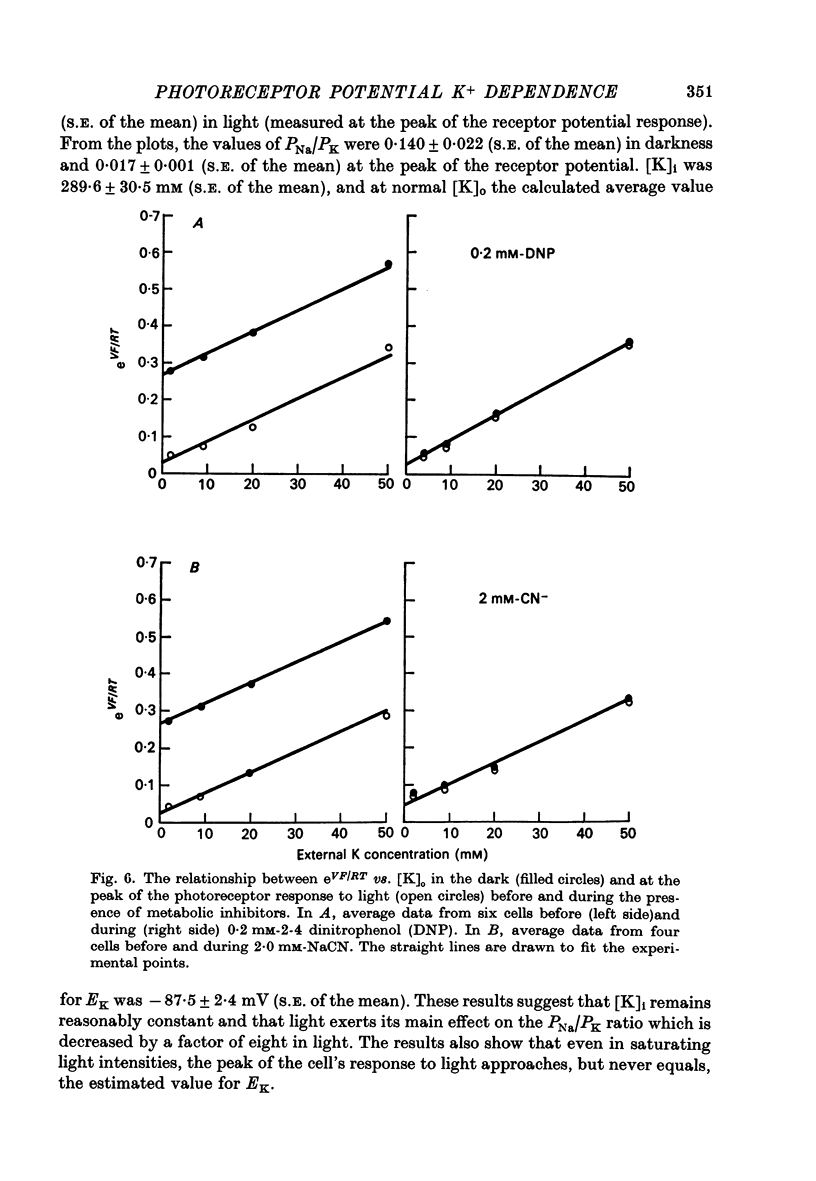
4. The ratio of Na+ to K+ permeabilities (PNa/PK) decreases during bright illumination. Our results suggest that PK is seven times that for PNa in the dark but is 57 times greater than PNa in light.
5. The metabolic inhibitors DNP and NaCN cause membrane potential in the dark to hyperpolarize. This hyperpolarization is associated with a decrease in the PNa/PK ratio similar to that found during illumination.
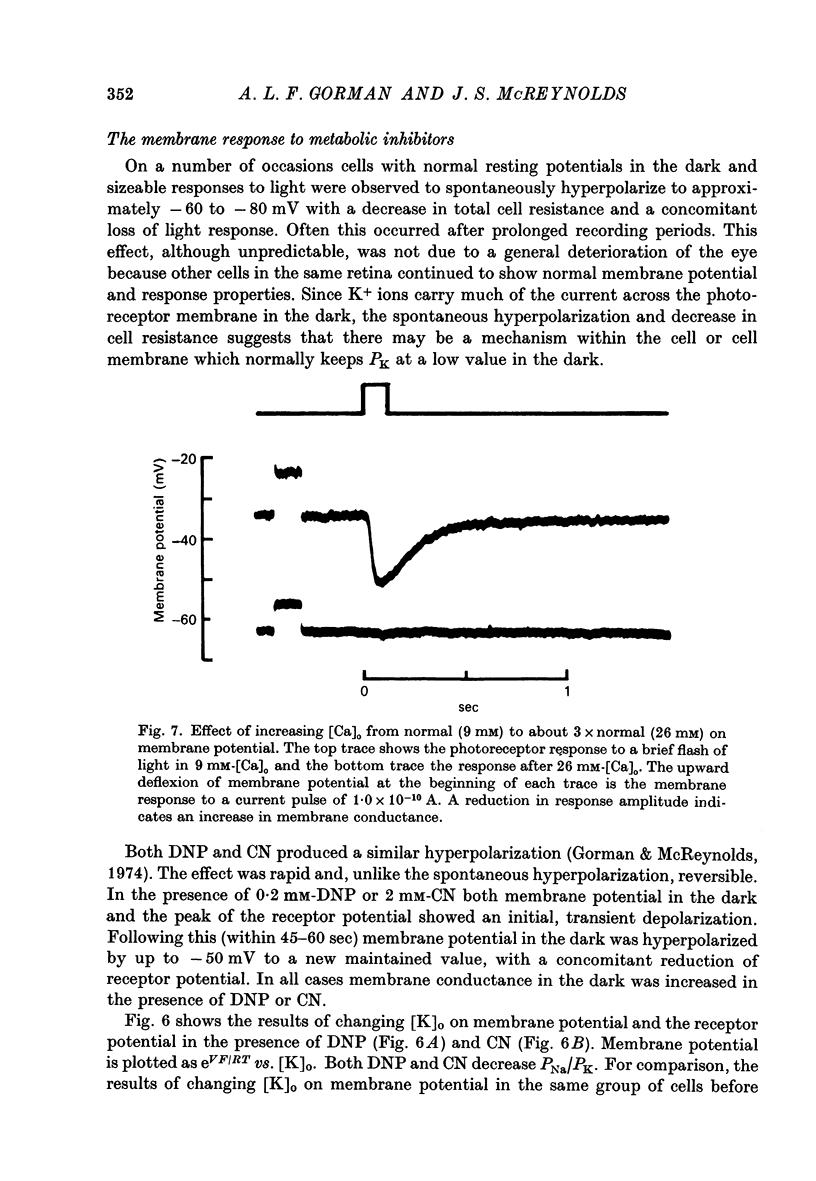
6. High [Ca+]o also causes membrane potential in the dark to hyperpolarize. This hyperpolarization is associated with an increase in membrane conductance.
7. The results indicate that the hyperpolarizing receptor potential of the distal photoreceptor is produced by a light-evoked increase in K+ permeability.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Barber V. C., Land M. F. Eye of the cockle, Cardium edule: anatomical and physiological investigations. Experientia. 1967 Aug 15;23(8):677–678. doi: 10.1007/BF02144199. [DOI] [PubMed] [Google Scholar]
- Bortoff A. Localization of slow potential responses in the Necturus retina. Vision Res. 1964 Dec;4(11):627–635. doi: 10.1016/0042-6989(64)90048-3. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Brown H. M. Light response of a giant Aplysia neuron. J Gen Physiol. 1973 Sep;62(3):239–254. doi: 10.1085/jgp.62.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Contributions of the sodium pump and ionic gradients to the membrane potential of a molluscan neurone. J Physiol. 1970 Nov;210(4):897–917. doi: 10.1113/jphysiol.1970.sp009248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., McReynolds J. S. Control of membrane N+ permeability in a hyperpolarizing photoreceptor: similar effect of light and metabolic inhibitors. Science. 1974 Aug 16;185(4151):620–621. doi: 10.1126/science.185.4151.620. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY D. Neural photoreception in a lamellibranch mollusc. J Gen Physiol. 1960 Nov;44:277–299. doi: 10.1085/jgp.44.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E., Rossi C. S. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- McReynolds J. S., Gorman A. L. Hyperpolarizing photoreceptors in the eye of a primitive chordate, Salpa democratica. Vision Res. 1975 Nov;15(11):1181–1186. doi: 10.1016/0042-6989(75)90160-1. [DOI] [PubMed] [Google Scholar]
- McReynolds J. S., Gorman A. L. Ionic basis of hyperpolarizing receptor potential in scallop eye: increase in permeability to potassium ions. Science. 1974 Feb 15;183(4125):658–659. doi: 10.1126/science.183.4125.658. [DOI] [PubMed] [Google Scholar]
- McReynolds J. S., Gorman A. L. Membrane conductances and spectral sensitivities of Pecten photoreceptors. J Gen Physiol. 1970 Sep;56(3):392–406. doi: 10.1085/jgp.56.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds J. S., Gorman A. L. Photoreceptor potentials of opposite polarity in the eye of the scallop, Pecten irradians. J Gen Physiol. 1970 Sep;56(3):376–391. doi: 10.1085/jgp.56.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreton R. B. An application of the constant-field theory to the behaviour of giant neurones of the snail, Helix aspersa. J Exp Biol. 1968 Jun;48(3):611–623. doi: 10.1242/jeb.48.3.611. [DOI] [PubMed] [Google Scholar]
- Mpitosos G. J. Physiology of vision in the mollusk Lima scabra. J Neurophysiol. 1973 Mar;36(2):371–383. doi: 10.1152/jn.1973.36.2.371. [DOI] [PubMed] [Google Scholar]
- Tabata M., Tamura T., Niwa H. Origin of the slow potential in the pineal organ of the rainbow trout. Vision Res. 1975 Jun;15(6):737–740. doi: 10.1016/0042-6989(75)90293-x. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrophysiological study of the mechanisms subserving color coding in the fish retina. Cold Spring Harb Symp Quant Biol. 1965;30:559–566. doi: 10.1101/sqb.1965.030.01.054. [DOI] [PubMed] [Google Scholar]
- Wiederhold M. L., MacNichol E. F., Jr, Bell A. L. Photoreceptor spike responses in the hardshell clam, Mercenaria mercenaria. J Gen Physiol. 1973 Jan;61(1):24–55. doi: 10.1085/jgp.61.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]


