Abstract
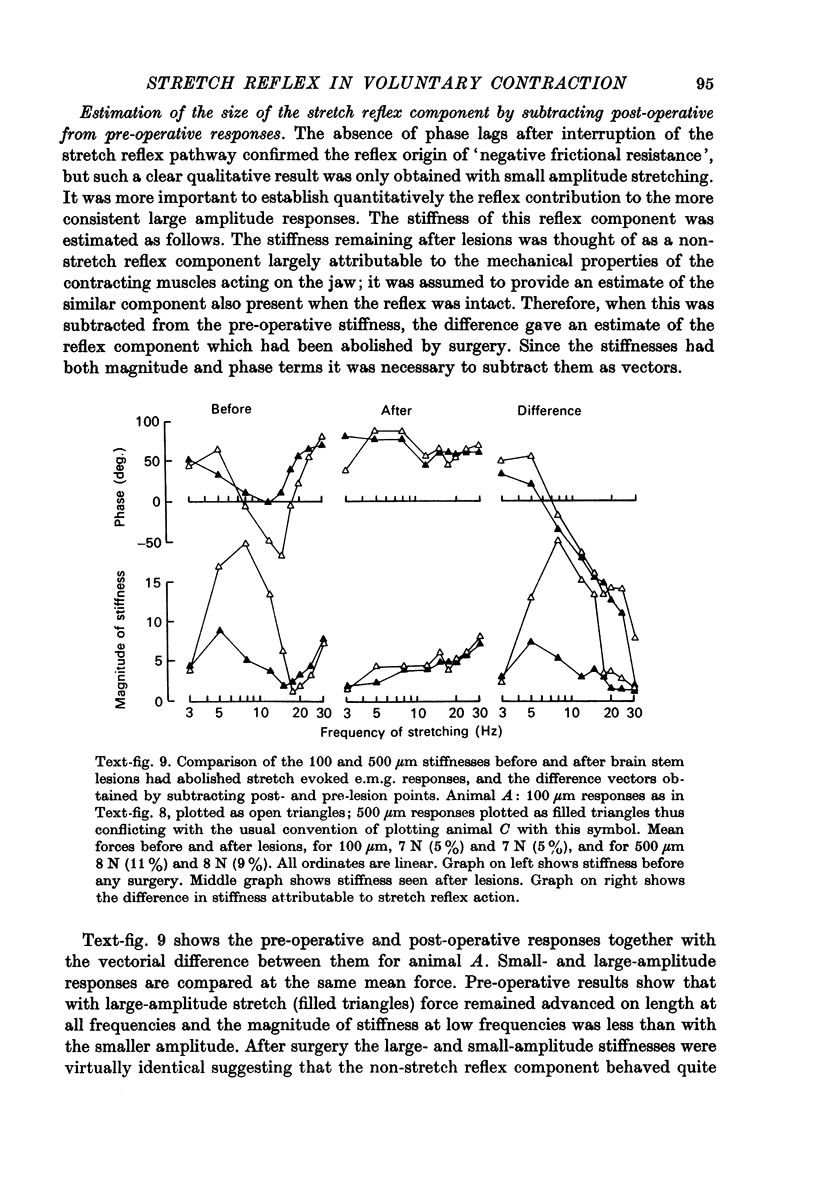
1. Rhesus monkeys were trained to exert steady biting forces of 3--60 N for 1--2 sec. This behaviour was well maintained while sinusoidal or step opening and closing movements were imposed on the jaw. 2. The amplitude of the force modulation during sinusoidal stretching was divided by the amplitude of movement to obtain the magnitude of stiffness. This estimate was made at frequencies from 2 to 50 Hz at amplitudes of 100 and 500 micrometer (half the peak-to-peak movement at the incisors). 3. Peak magnitudes of stiffness were seen with frequencies of 8--15 Hz when the amplitude of movement was small; there was a great deal of variation between individual animals. This variation was most striking with mean forces of 25--35 N. The stiffness was greatest in animals that showed considerable spontaneous tremor, and the highest levels of stiffness were often recorded with frequencies near which tremor amplitude was large. A marked phase lag in the force response was often seen during small amplitude stretching at 8--30 Hz. 4. Estimates of stiffness for larger amplitude (500 micrometer) stretching showed less variation; the magnitude of stiffness showed maximum values below 10 Hz and a minimum at 15--30 Hz. Force always showed a phase lead on position although this lead became small in the frequency range where with smaller movement there had been phase lags. The magnitude of stiffness increased with increasing mean force. 5. Bilateral electrolytic lesions were made in the brain stems of three animals; they reduced by over 95% the expected number of cells in the mesencephalic nucleus of the fifth cranial nerve on either side. These lesions interrupted the afferent pathway for the stretch reflex and so abolished excitatory electromyogram (e.m.g.) responses to step stretches of the jaw closing muscles. 6. Such reflex responses as persisted after the lesions were small and inhibitory. E.m.g. silences followed both step stretch and release; the response to release was a 'load compensation' that could not be attributed to spindle afferents. 7. After the lesions the responses to movements of 100 micrometer showed neither negative values for the phase nor marked peaks in the stiffness magnitude at low frequencies; these features therefore take origin in the action of the stretch reflex. The stiffness that was measured after the lesions may be attributed to the non-reflex components resisting stretch, particularly to the properties of the contracting muscles. Thus, the phase of the force response was markedly advanced at all frequencies and the stiffness seen for 100 micrometer was similar to that for 500 micrometer. Stiffness increased with increasing mean force, as before surgery. 8. Vector subtraction of the stiffness seen at each frequency after interrupting the stretch reflex from that seen before doing so gave a quantitative estimate of the strength of the stretch reflex. The reflex activity calculated in this way showed attenuation and progressive phase lag as the frequency increased above 10 Hz...
Full text
PDF





























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
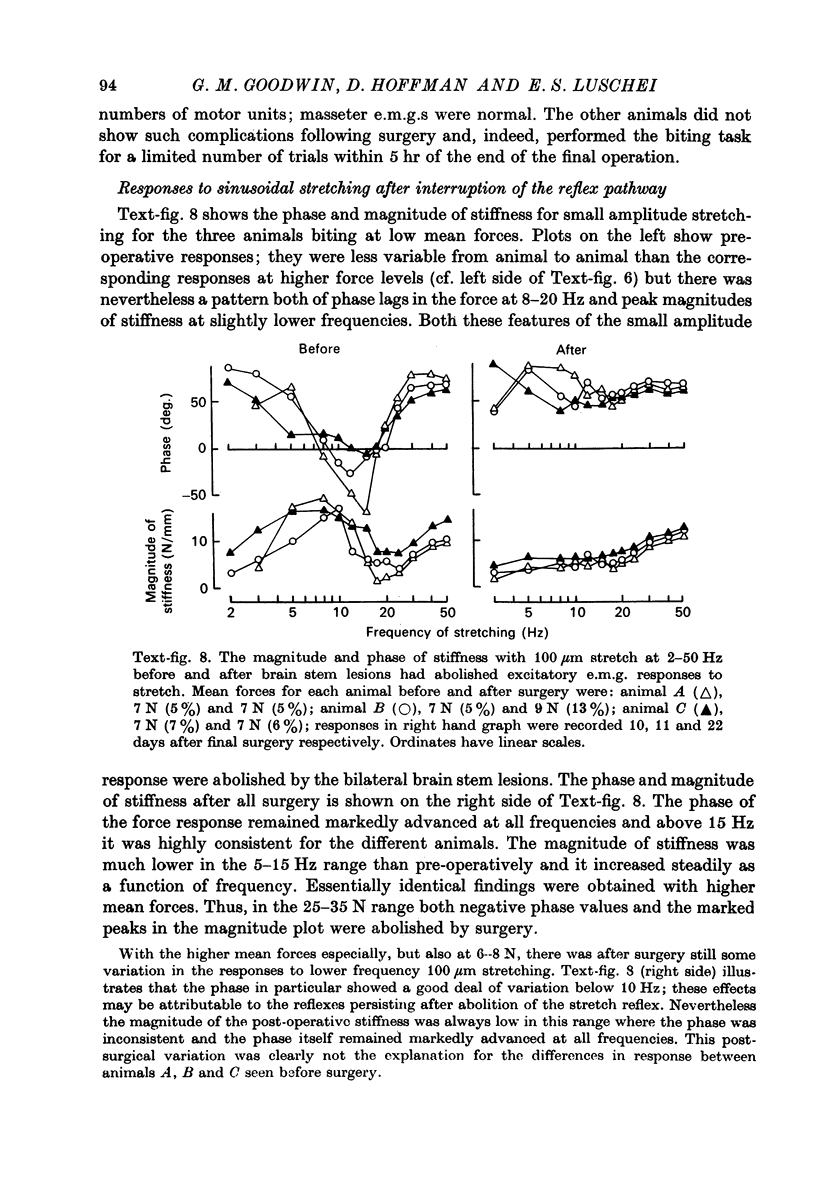
- ABBOTT B. C., AUBERT X. M. The force exerted by active striated muscle during and after change of length. J Physiol. 1952 May;117(1):77–86. [PMC free article] [PubMed] [Google Scholar]
- Agarwal G. C., Gottlieb G. L. Oscillation of the human ankle joint in response to applied sinusoidal torque on the foot. J Physiol. 1977 Jun;268(1):151–176. doi: 10.1113/jphysiol.1977.sp011852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander R. M., Bennet-Clark H. C. Storage of elastic strain energy in muscle and other tissues. Nature. 1977 Jan 13;265(5590):114–117. doi: 10.1038/265114a0. [DOI] [PubMed] [Google Scholar]
- Angel R. W., Eppler W., Iannone A. Silent period produced by unloading of muscle during voluntary contraction. J Physiol. 1965 Oct;180(4):864–870. doi: 10.1113/jphysiol.1965.sp007736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa P., Mannard A., Stein R. B. Effects of elastic loads on the contractions of cat muscles. Biol Cybern. 1976;22(3):129–137. doi: 10.1007/BF00365523. [DOI] [PubMed] [Google Scholar]
- Bratzlavsky M. Pauses in activity of human jaw closing muscle. Exp Neurol. 1972 Jul;36(1):160–165. doi: 10.1016/0014-4886(72)90143-4. [DOI] [PubMed] [Google Scholar]
- Cody F. W., Lee R. W., Taylor A. A functional analysis of the components of the mesencephalic nucleus of the fifth nerve in the cat. J Physiol. 1972 Oct;226(1):249–261. doi: 10.1113/jphysiol.1972.sp009983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago P. E., Houk J. C., Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol. 1976 Sep;39(5):925–935. doi: 10.1152/jn.1976.39.5.925. [DOI] [PubMed] [Google Scholar]
- Davis J. N., Sears T. A. The proprioceptive reflex control of the intercostal muscles during their voluntary activation. J Physiol. 1970 Aug;209(3):711–738. doi: 10.1113/jphysiol.1970.sp009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elble R. J., Randall J. E. Motor-unit activity responsible for 8- to 12-Hz component of human physiological finger tremor. J Neurophysiol. 1976 Mar;39(2):370–383. doi: 10.1152/jn.1976.39.2.370. [DOI] [PubMed] [Google Scholar]
- Furness P., Jessop J., Lippold O. C. Long-lasting increases in the tremor of human hand muscles following brief, strong effort. J Physiol. 1977 Mar;265(3):821–831. doi: 10.1113/jphysiol.1977.sp011746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg L. J. Masseter muscle excitation induced by stimulation of periodontal and gingival receptors in man. Brain Res. 1971 Sep 24;32(2):369–381. doi: 10.1016/0006-8993(71)90330-1. [DOI] [PubMed] [Google Scholar]
- Goodwin G. M., Hoffman D. S., Luschei E. S. Stretch reflex strength in voluntary biting revealed following electrolytic lesions of monkey mesencephalic nucleus (5th cranial nerve) [proceedings]. J Physiol. 1977 Jul;269(1):53P–54P. [PubMed] [Google Scholar]
- Goodwin G. M., Hulliger M., Matthews P. B. The effects of fusimotor stimulation during small amplitude stretching on the frequency-response of the primary ending of the mammalian muscle spindle. J Physiol. 1975 Dec;253(1):175–206. doi: 10.1113/jphysiol.1975.sp011186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. M., Luschei E. S. Effects of destroying spindle afferents from jaw muscles on mastication in monkeys. J Neurophysiol. 1974 Sep;37(5):967–981. doi: 10.1152/jn.1974.37.5.967. [DOI] [PubMed] [Google Scholar]
- HALLIDAY A. M., REDFEARN J. W. An analysis of the frequencies of finger tremor in healthy subjects. J Physiol. 1956 Dec 28;134(3):600–611. doi: 10.1113/jphysiol.1956.sp005668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLIDAY A. M., REDFEARN J. W. Finger tremor in tabetic patients and its bearing on the mechanism producing the rhythm of physiological tremor. J Neurol Neurosurg Psychiatry. 1958 May;21(2):101–108. doi: 10.1136/jnnp.21.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMMOND P. H., MERTON P. A., SUTTON G. G. Nervous gradation of muscular contraction. Br Med Bull. 1956 Sep;12(3):214–218. doi: 10.1093/oxfordjournals.bmb.a069553. [DOI] [PubMed] [Google Scholar]
- HUGELIN A., BONVALLET M. Etude électrophysiologique d'un réflexe monosynaptique trigéminal. C R Seances Soc Biol Fil. 1956;150(12):2067–2071. [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- JERGE C. R. Organization and function of the trigeminal mensencephalic nucleus. J Neurophysiol. 1963 May;26:379–392. doi: 10.1152/jn.1963.26.3.379. [DOI] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M., Ross H. F. The forces generated at the human elbow joint in response to imposed sinusoidal movements of the forearm. J Physiol. 1974 Jul;240(2):351–374. doi: 10.1113/jphysiol.1974.sp010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M. The effects of load and force on tremor at the normal human elbow joint. J Physiol. 1974 Jul;240(2):375–396. doi: 10.1113/jphysiol.1974.sp010615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M., Westbury D. R. The mechanical properties of cat soleus muscle during controlled lengthening and shortening movements. J Physiol. 1969 Oct;204(2):461–474. doi: 10.1113/jphysiol.1969.sp008924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y., Kubota K., Shuto S., Sumino R. Reflex organization of cat masticatory muscles. J Neurophysiol. 1968 Sep;31(5):695–708. doi: 10.1152/jn.1968.31.5.695. [DOI] [PubMed] [Google Scholar]
- LIPPOLD O. C., REDFEARN J. W., VUCO J. The rhythmical activity of groups of motor units in the voluntary contraction of muscle. J Physiol. 1957 Aug 6;137(3):473–487. doi: 10.1113/jphysiol.1957.sp005828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEWENSTEIN W. R., MENDELSON M. COMPONENTS OF RECEPTOR ADAPTATION IN A PACINIAN CORPUSCLE. J Physiol. 1965 Apr;177:377–397. doi: 10.1113/jphysiol.1965.sp007598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarre Y., Lund J. P. Load compensation in human masseter muscles. J Physiol. 1975 Dec;253(1):21–35. doi: 10.1113/jphysiol.1975.sp011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippold O. C. Oscillation in the stretch reflex arc and the origin of the rhythmical, 8-12 C-S component of physiological tremor. J Physiol. 1970 Feb;206(2):359–382. doi: 10.1113/jphysiol.1970.sp009018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschei E. S., Goodwin G. M. Patterns of mandibular movement and jaw muscle activity during mastication in the monkey. J Neurophysiol. 1974 Sep;37(5):954–966. doi: 10.1152/jn.1974.37.5.954. [DOI] [PubMed] [Google Scholar]
- MARSHALL J., WALSH E. G. Physiological tremor. J Neurol Neurosurg Psychiatry. 1956 Nov;19(4):260–267. doi: 10.1136/jnnp.19.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannard A., Stein R. B. Determination of the frequency response of isometric soleus muscle in the cat using random nerve stimulation. J Physiol. 1973 Mar;229(2):275–296. doi: 10.1113/jphysiol.1973.sp010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Servo action in human voluntary movement. Nature. 1972 Jul 21;238(5360):140–143. doi: 10.1038/238140a0. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Stretch reflex and servo action in a variety of human muscles. J Physiol. 1976 Jul;259(2):531–560. doi: 10.1113/jphysiol.1976.sp011481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. The sensory mechanism of servo action in human muscle. J Physiol. 1977 Feb;265(2):521–535. doi: 10.1113/jphysiol.1977.sp011728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Stein R. B. The sensitivity of muscle spindle afferents to small sinusoidal changes of length. J Physiol. 1969 Feb;200(3):723–743. doi: 10.1113/jphysiol.1969.sp008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. The dependence of tension upon extension in the stretch reflex of the soleus muscle of the decerebrate cat. J Physiol. 1959 Oct;147(3):521–546. doi: 10.1113/jphysiol.1959.sp006260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McINTYRE A. K. Afferent limb of the myotatic reflex arc. Nature. 1951 Jul 28;168(4265):168–169. doi: 10.1038/168168a0. [DOI] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Lee R. G. Synchronization of human motor units: possible roles of exercise and supraspinal reflexes. Electroencephalogr Clin Neurophysiol. 1975 Mar;38(3):245–254. doi: 10.1016/0013-4694(75)90245-x. [DOI] [PubMed] [Google Scholar]
- Neilson P. D. Speed of response or bandwidth of voluntary system controlling elbow position in intact man. Med Biol Eng. 1972 Jul;10(4):450–459. doi: 10.1007/BF02474193. [DOI] [PubMed] [Google Scholar]
- Nichols T. R., Houk J. C. Improvement in linearity and regulation of stiffness that results from actions of stretch reflex. J Neurophysiol. 1976 Jan;39(1):119–142. doi: 10.1152/jn.1976.39.1.119. [DOI] [PubMed] [Google Scholar]
- PARTRIDGE L. D. MODIFICATIONS OF NEURAL OUTPUT SIGNALS BY MUSCLES: A FREQUENCY RESPONSE STUDY. J Appl Physiol. 1965 Jan;20:150–156. doi: 10.1152/jappl.1965.20.1.150. [DOI] [PubMed] [Google Scholar]
- Poppele R. E., Terzuolo C. A. Myotatic reflex: its input-output relation. Science. 1968 Feb 16;159(3816):743–745. doi: 10.1126/science.159.3816.743. [DOI] [PubMed] [Google Scholar]
- Rack P. M., Westbury D. R. The effects of length and stimulus rate on tension in the isometric cat soleus muscle. J Physiol. 1969 Oct;204(2):443–460. doi: 10.1113/jphysiol.1969.sp008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack P. M., Westbury D. R. The short range stiffness of active mammalian muscle and its effect on mechanical properties. J Physiol. 1974 Jul;240(2):331–350. doi: 10.1113/jphysiol.1974.sp010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno A., Stein R. B., Purple R. L., Poppele R. E. A neuronal model for the discharge patterns produced by cyclic inputs. Bull Math Biophys. 1970 Sep;32(3):337–353. doi: 10.1007/BF02476873. [DOI] [PubMed] [Google Scholar]
- Rosenthal N. P., McKean T. A., Roberts W. J., Terzuolo C. A. Frequency analysis of stretch reflex and its main subsystems in triceps surae muscles of the cat. J Neurophysiol. 1970 Nov;33(6):713–749. doi: 10.1152/jn.1970.33.6.713. [DOI] [PubMed] [Google Scholar]
- Sherrington C. S. Reflexes elicitable in the cat from pinna vibrissae and jaws. J Physiol. 1917 Dec 6;51(6):404–431. doi: 10.1113/jphysiol.1917.sp001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. B., French A. S., Holden A. V. The frequency response, coherence, and information capacity of two neuronal models. Biophys J. 1972 Mar;12(3):295–322. doi: 10.1016/S0006-3495(72)86087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. B. Peripheral control of movement. Physiol Rev. 1974 Jan;54(1):215–243. doi: 10.1152/physrev.1974.54.1.215. [DOI] [PubMed] [Google Scholar]
- Sutton G. G., Sykes K. The effect of withdrawal of visual presentation of errors upon the frequency spectrum of tremor in a manual task. J Physiol. 1967 May;190(2):281–293. doi: 10.1113/jphysiol.1967.sp008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR A. The significance of grouping of motor unit activity. J Physiol. 1962 Jul;162:259–269. doi: 10.1113/jphysiol.1962.sp006930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo A. B. Human muscle spindle discharge during isometric voluntary contractions. Amplitude relations between spindle frequency and torque. Acta Physiol Scand. 1974 Feb;90(2):319–336. doi: 10.1111/j.1748-1716.1974.tb05594.x. [DOI] [PubMed] [Google Scholar]