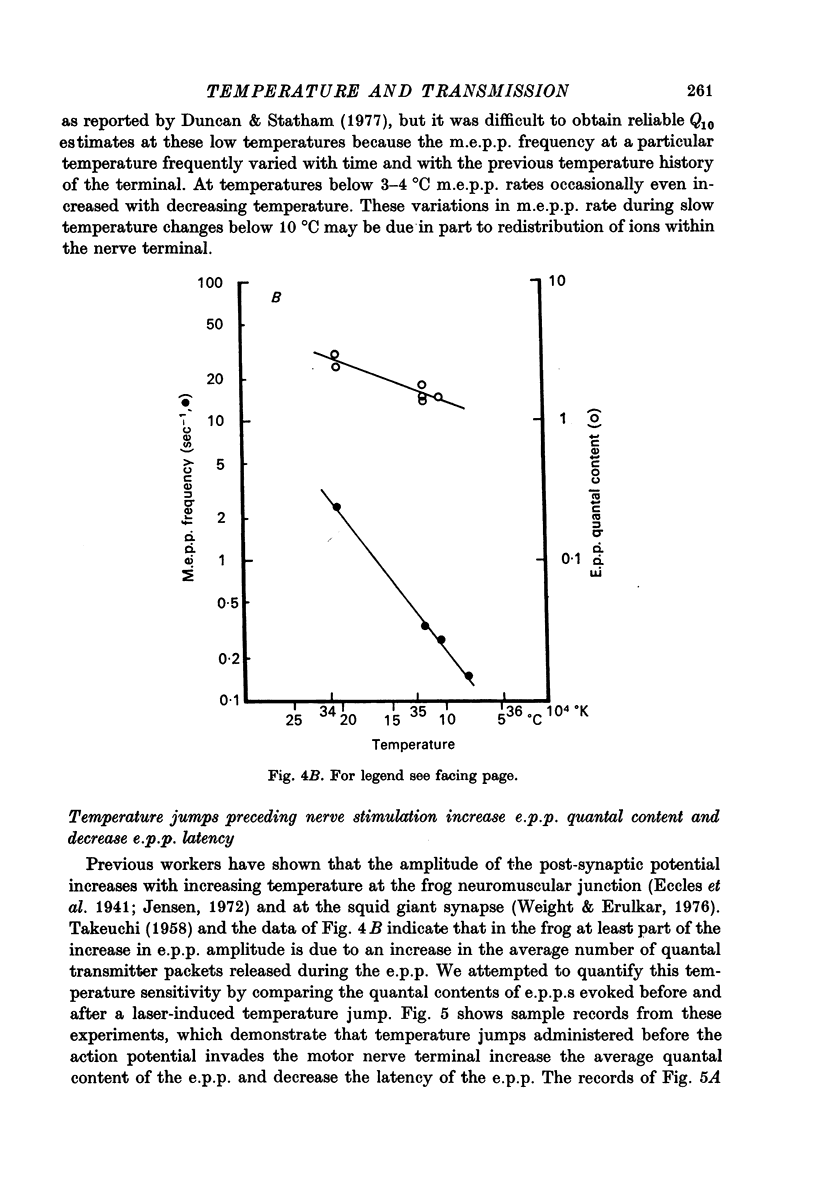
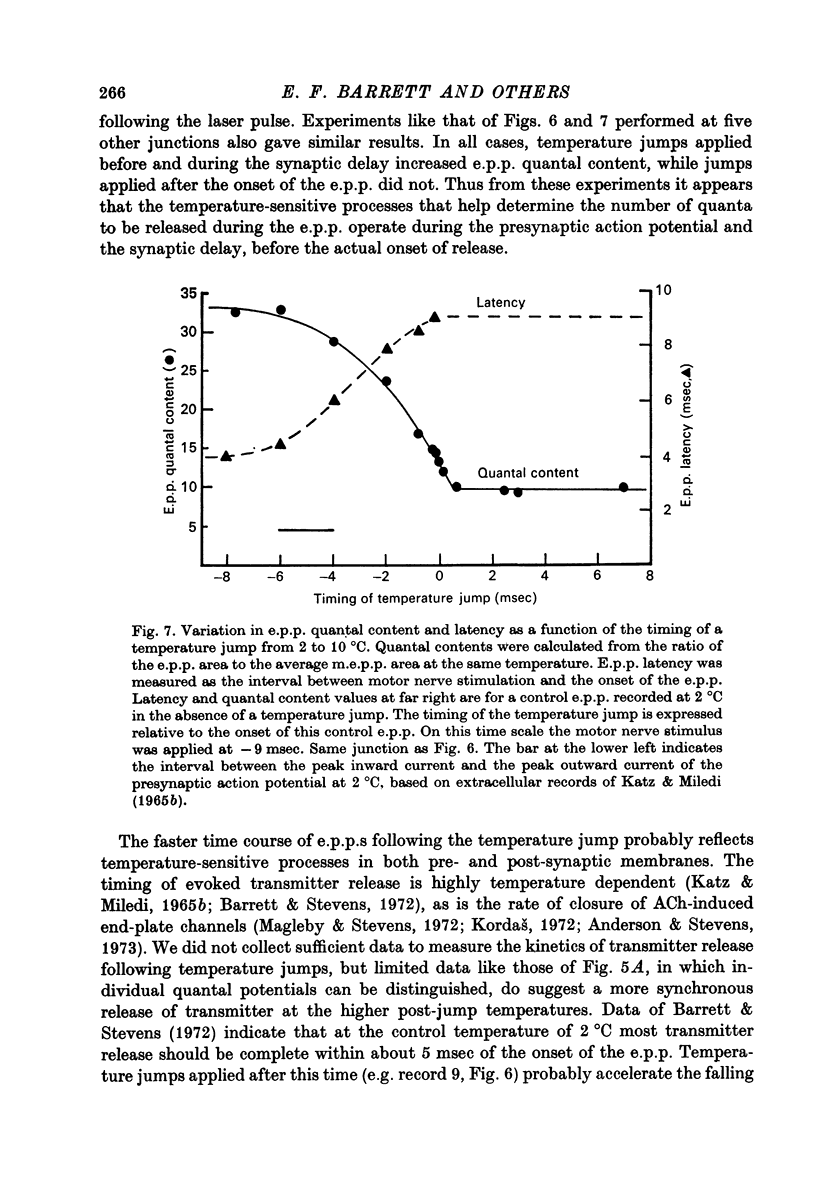
Abstract
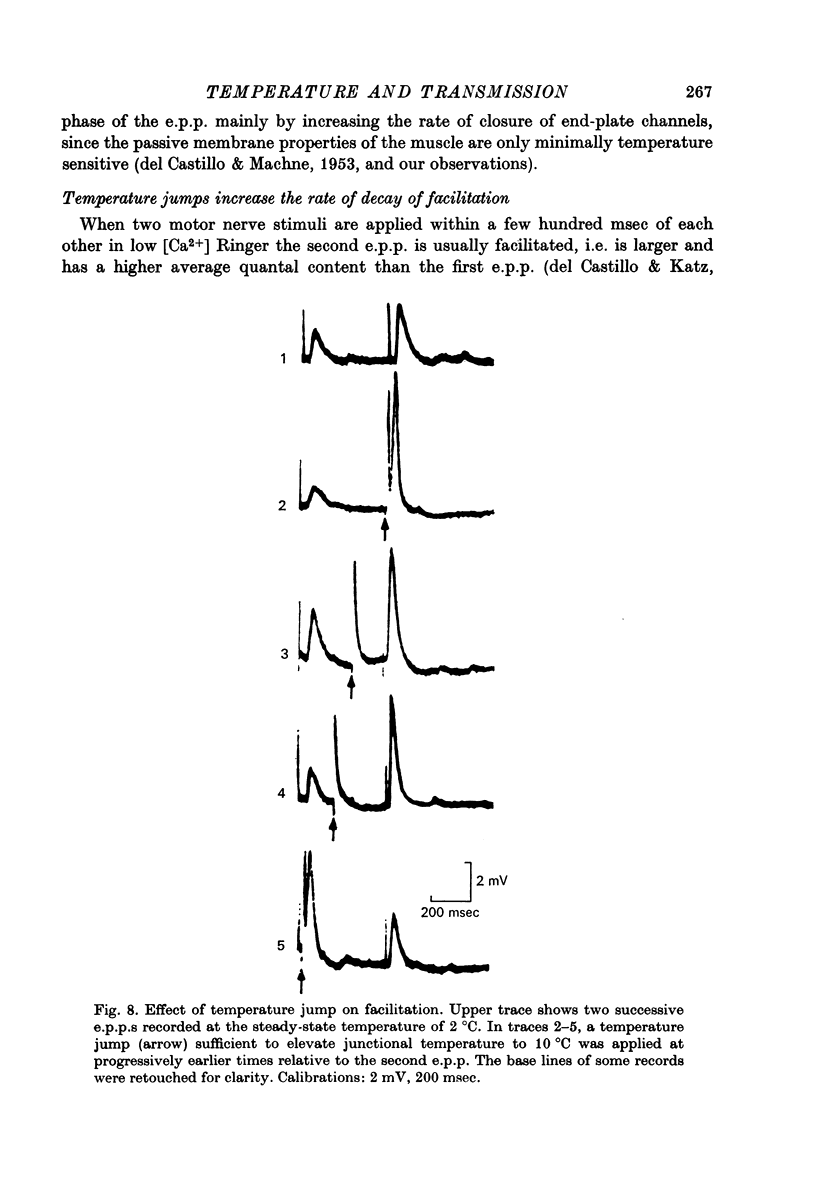
1. The temperature dependence of presynaptic processes involved in neuromuscular transmission was studied by rapidly increasing the temperature of cooled frog neuromuscular junctions by 4--10 degrees C using pulses from a neodymium laser. The temperature elevation was complete within 0.5 msec, and decayed back to control levels with a time constant of about 7--8 sec. 2. Temperature jumps completed before nerve stimulation increased the quantal content and decreased the latency of the end-plate potential (e.p.p.). The Q10 for e.p.p. quantal content in low [Ca2+] Ringer averaged about 3.9 over the range 1--18 degrees C. 3. Temperature jumps occurring during the synaptic delay (the interval between the presynaptic action potential and the onset of the e.p.p.) also increased the quantal content and decreased the latency of the e.p.p. These effects diminished as the onset of the temperature jump was moved closer to the expected onset of the e.p.p. Temperature jumps applied after the onset of the e.p.p. immediately accelerated the time course of the e.p.p. but did not significantly alter quantal content. These results demonstrate that the magnitude and timing of evoked release are influenced by temperature-sensitive processes that operate both during and shortly after the presynaptic nerve action potential, but are largely complete before the onset of release. 4. Temperature jumps were applied at various times during the interval between two nerve stimuli. The amplitude of the second e.p.p. decreased as the temperature jump was moved earlier in the interstimulus interval, suggesting that the rise in temperature following the first nerve stimulus accelerates the decay of facilitation. When the temperature jump was moved from 10 msec after to 10 msec before the onset of the first e.p.p., the amplitude of the second e.p.p. either decreased or showed no change. The fact that the second e.p.p. did not increase suggests that the temperature-sensitive processes that increase the quantal content of the conditioning e.p.p. do not greatly increase the facilitation following that e.p.p. 5. Temperature jumps immediately accelerated the time course of spontaneous miniature end-plate potentials (m.e.p.p.s) and increased their frequency. Experiments using slow temperature changes revealed that the Q10 for m.e.p.p. frequency in normal Ringer is about 10 over the range 10--20 degrees C. M.e.p.p. frequency was much less sensitive to temperature changes below about 10 degrees C. When the nerve terminal was depolarized by 20 mM-K+ in the presence of Ca2+, the Q10 for the rate of spontaneous release over the range 10--20 degrees C decreased to about 4, similar to the Q10 for e.p.p. quantal content. In the absence of extracellular Ca2+ the Q10 for m.e.p.p. frequency in 20 mM-K+ remained near 10. 6. The marked difference in Q10S for spontaneous transmitter release under different experimental conditions suggests that not all transmitter release uses identical mechanisms...
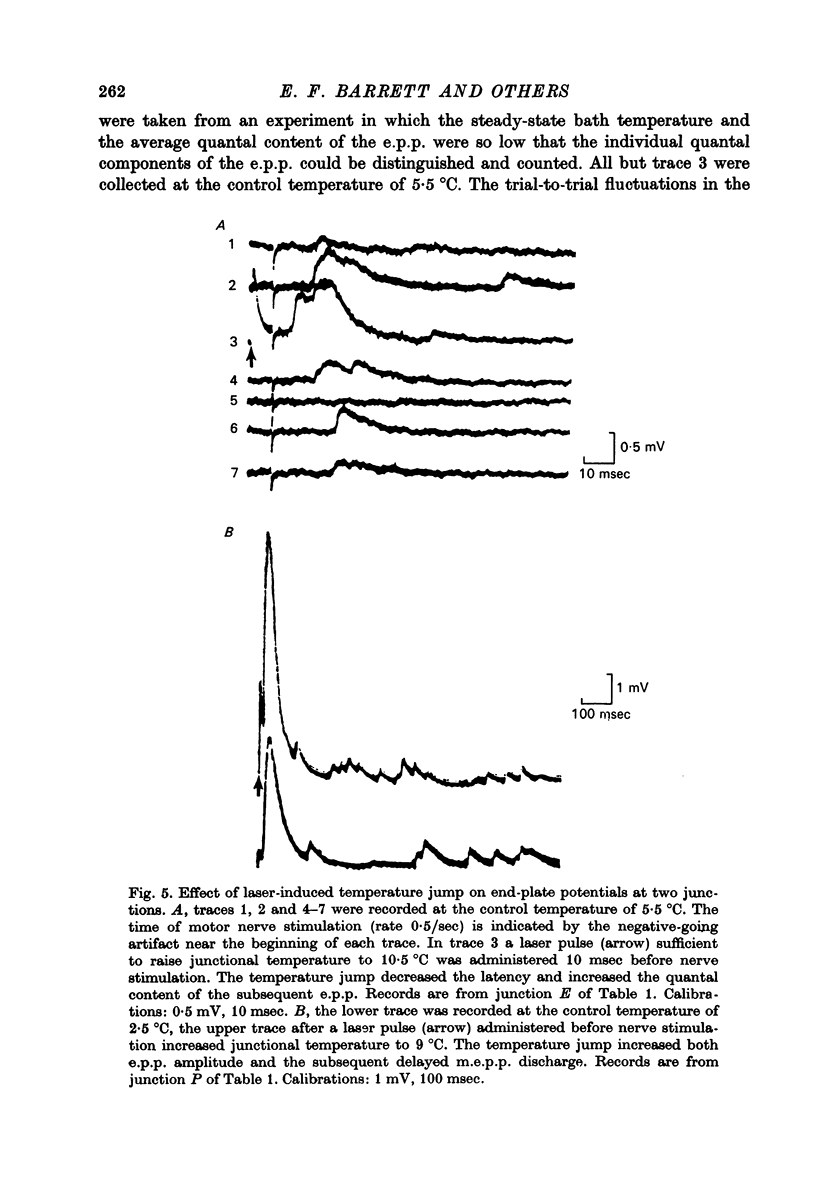
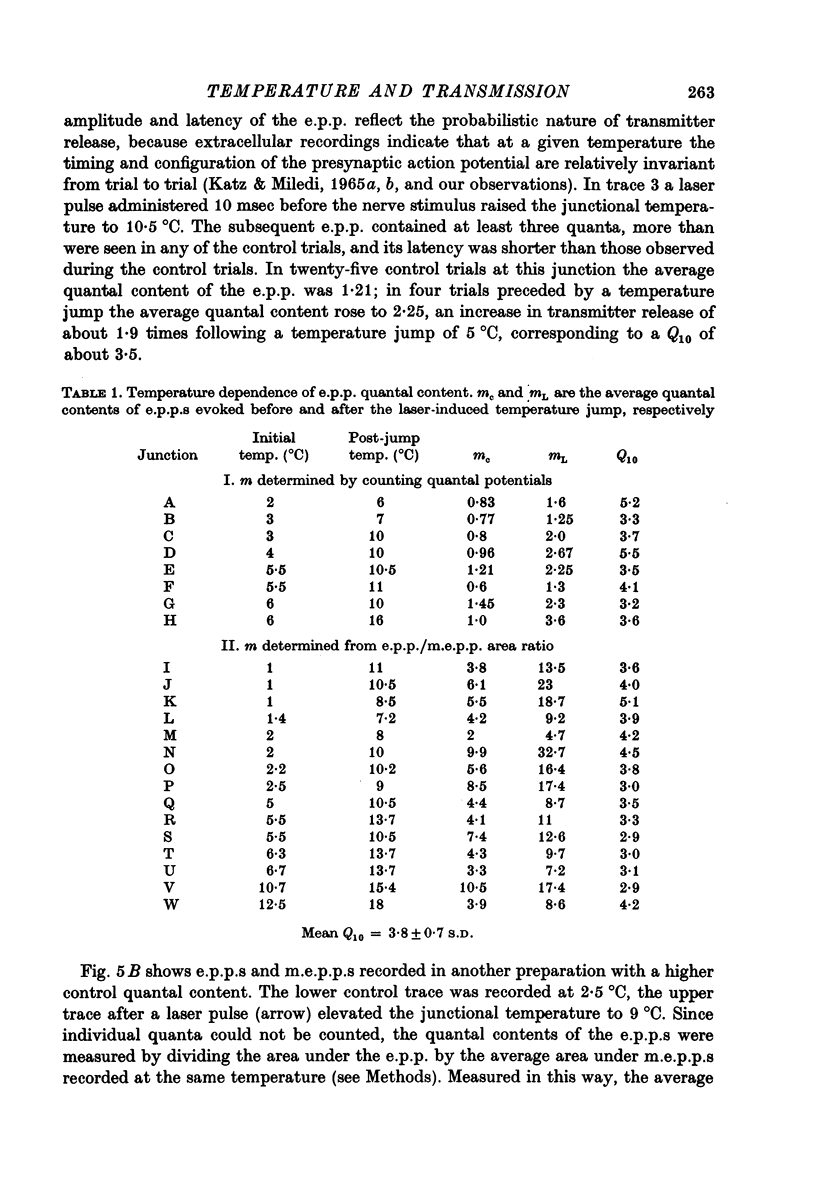
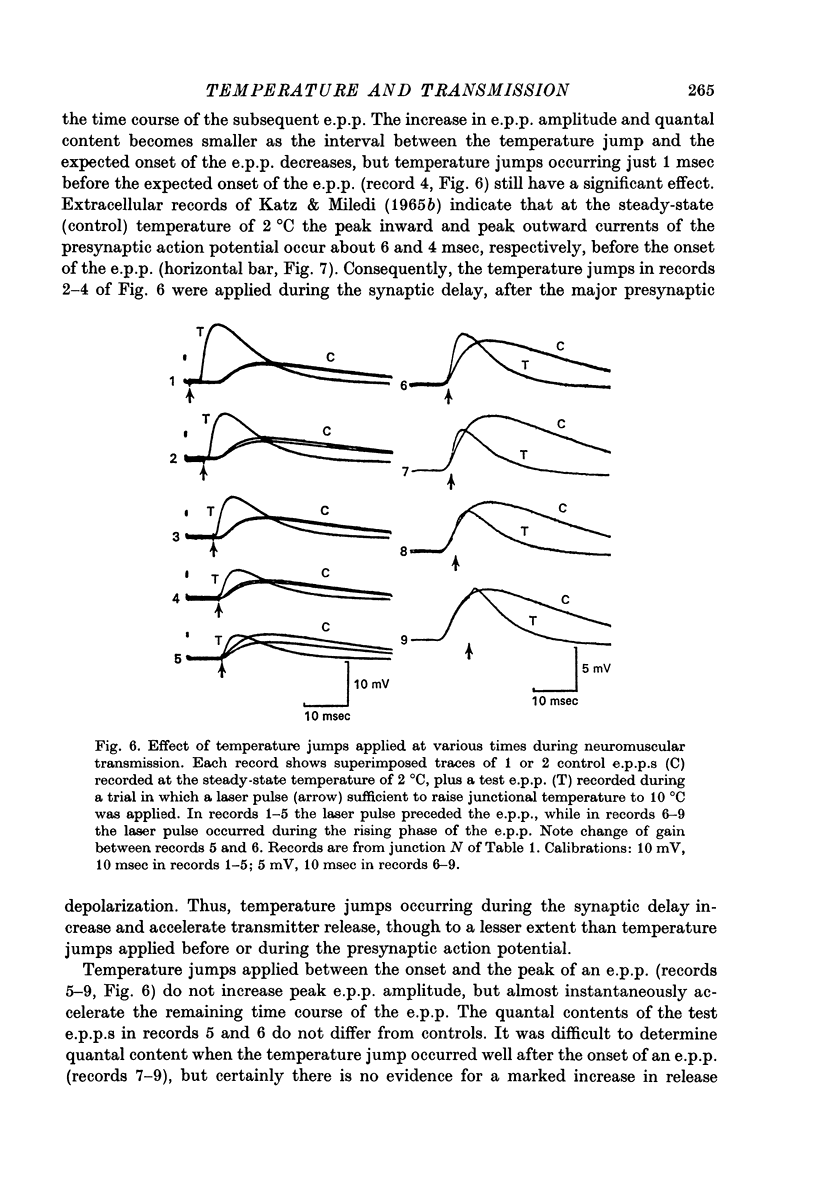
Full text
PDF


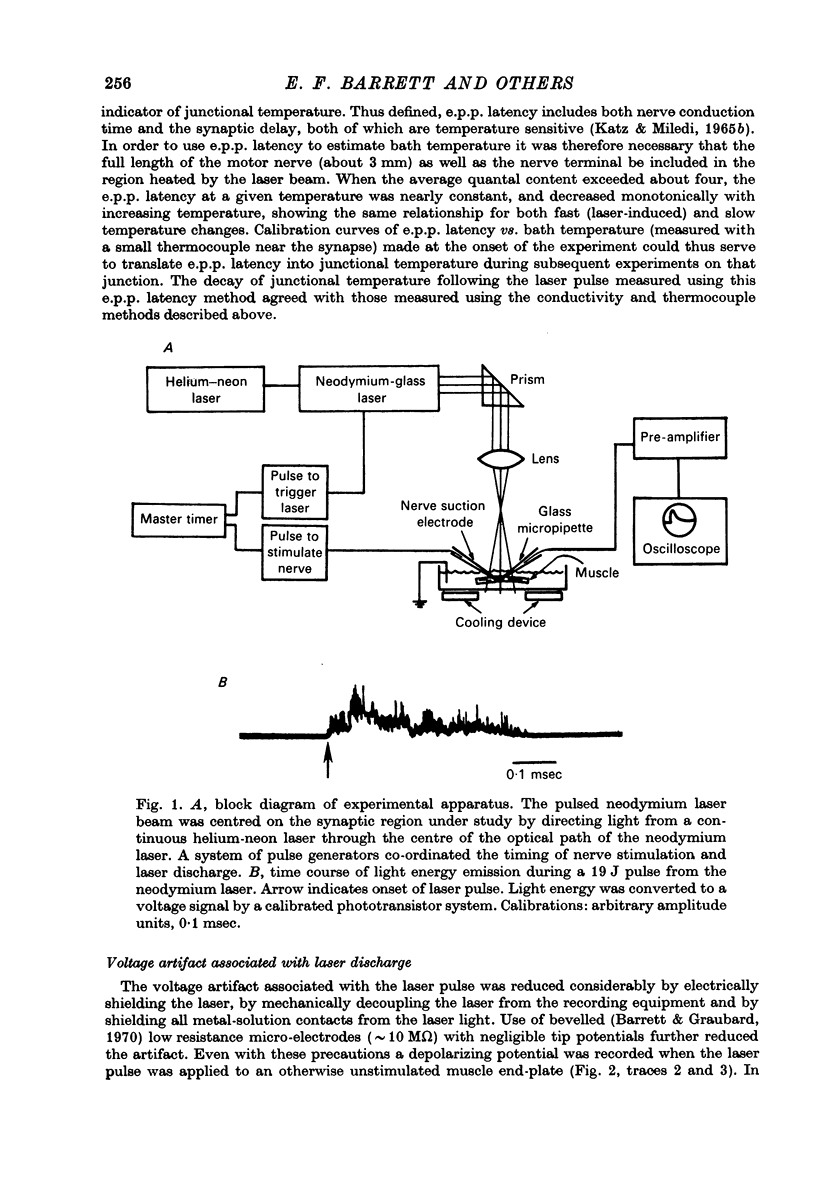
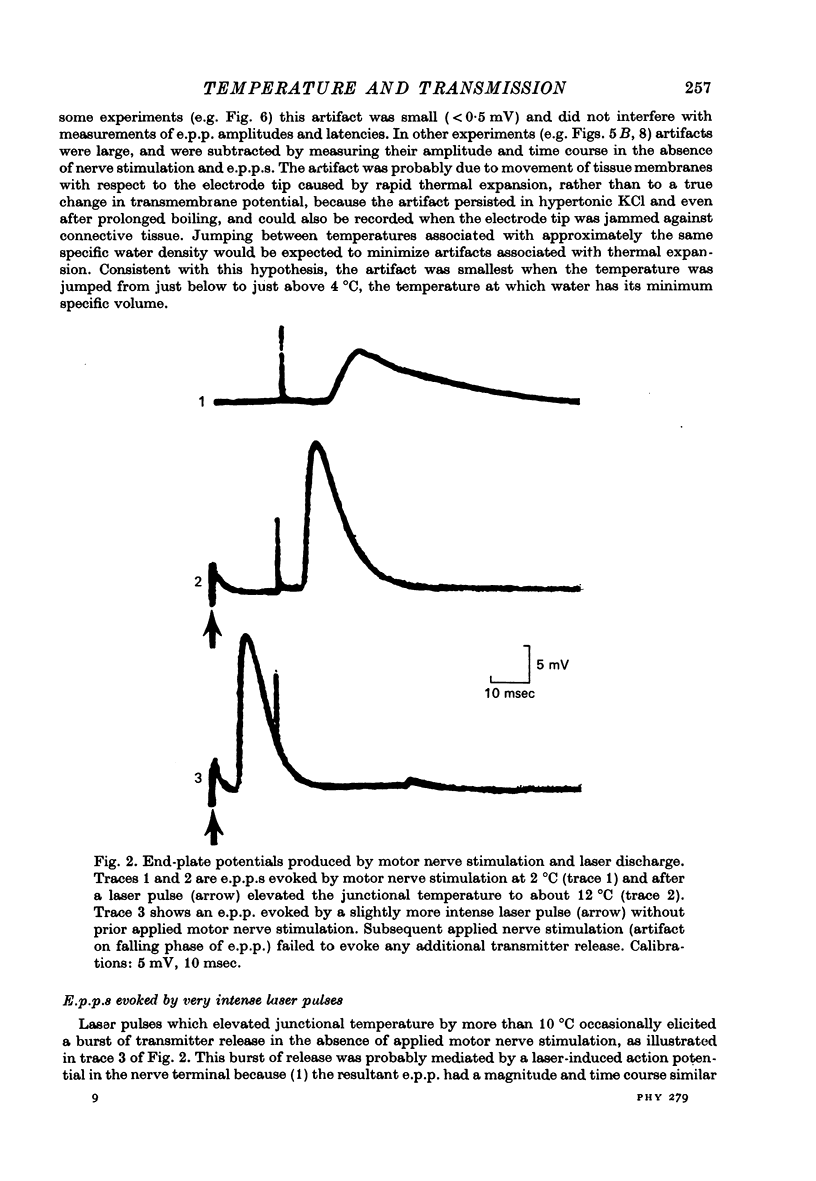

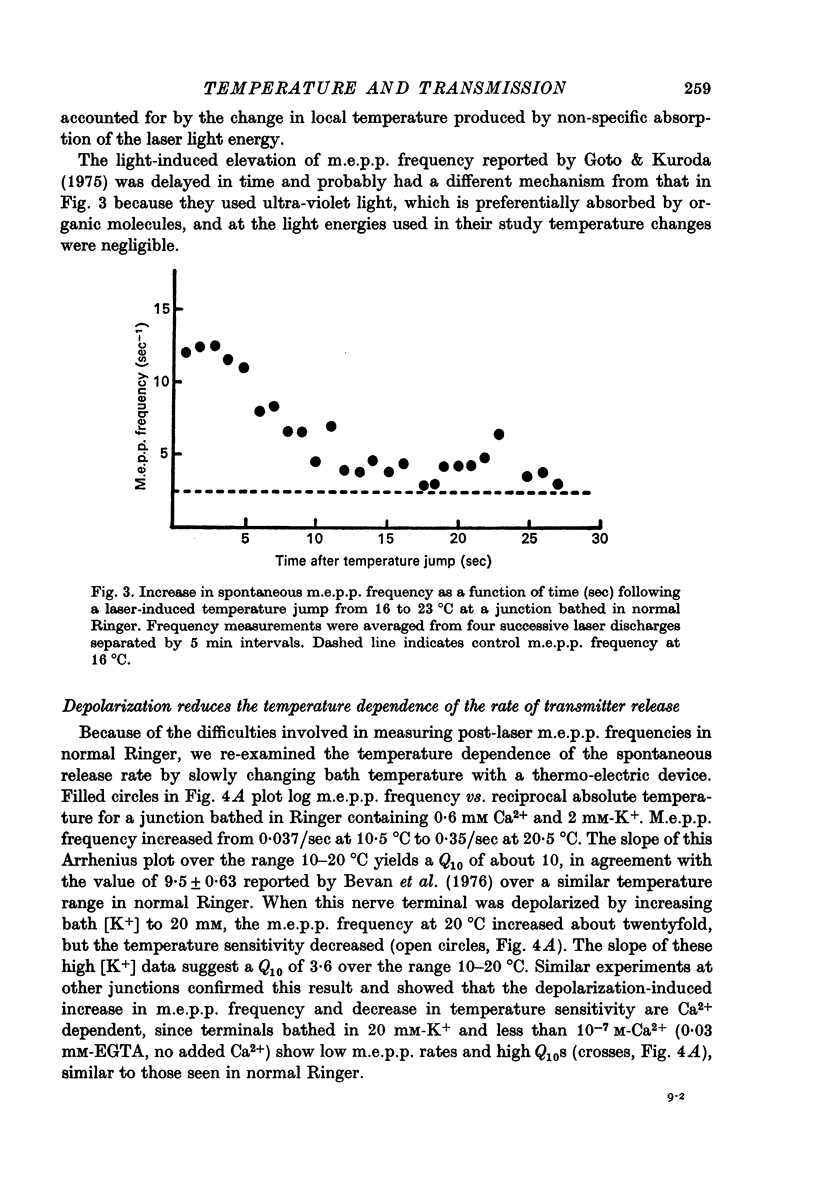
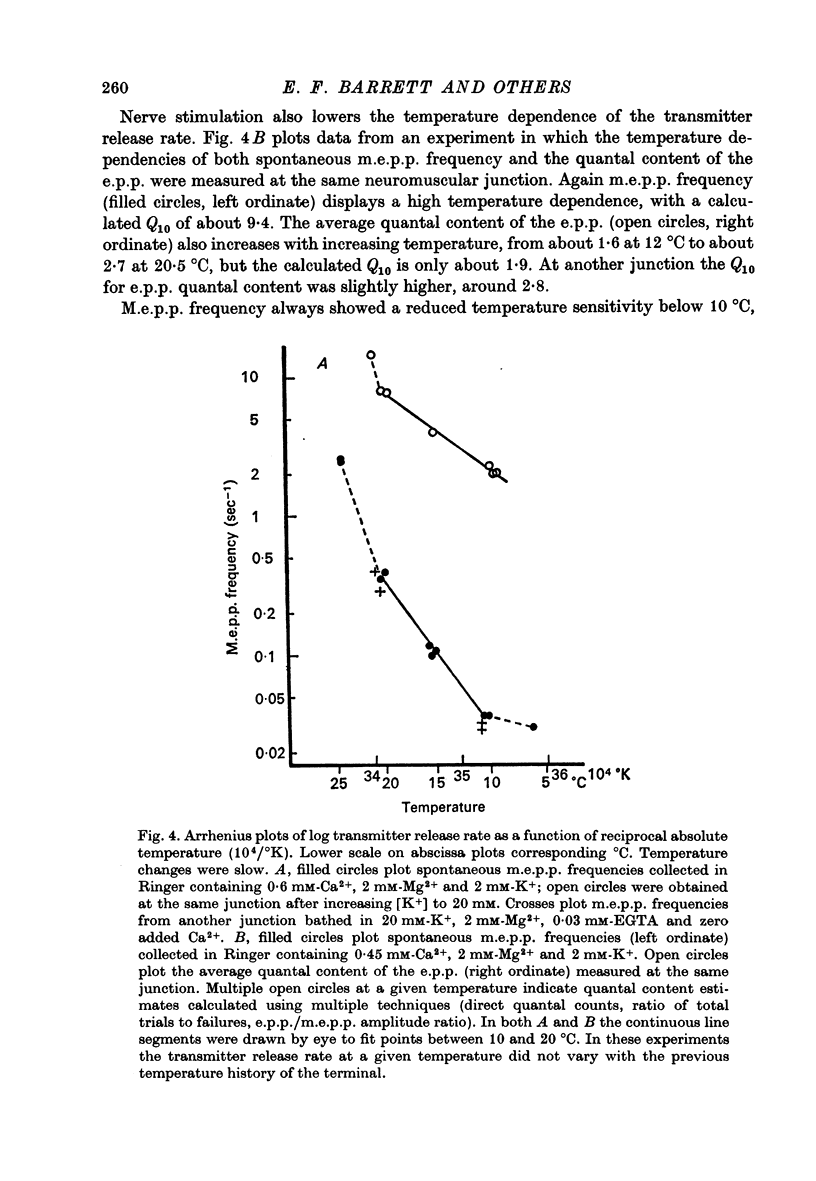







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. Spontaneous subthreshold activity at mammalian neural muscular junctions. J Physiol. 1956 Apr 27;132(1):61–73. doi: 10.1113/jphysiol.1956.sp005502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balnave R. J., Gage P. W. On facilitation of transmitter release at the toad neuromuscular junction. J Physiol. 1974 Jun;239(3):657–675. doi: 10.1113/jphysiol.1974.sp010588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. The kinetics of transmitter release at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):691–708. doi: 10.1113/jphysiol.1972.sp010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Graubard K. Fluorescent staining of cat motoneurons in vivo with beveled micropipettes. Brain Res. 1970 Mar 17;18(3):565–568. doi: 10.1016/0006-8993(70)90143-5. [DOI] [PubMed] [Google Scholar]
- Bevan S., Grampp W., Miledi R. Properties of spontaneous potentials at denervated motor endplates of the frog. Proc R Soc Lond B Biol Sci. 1976 Oct 15;194(1115):195–210. doi: 10.1098/rspb.1976.0073. [DOI] [PubMed] [Google Scholar]
- Cooke J. D., Okamoto K., Quastel D. M. The role of calcium in depolarization-secretion coupling at the motor nerve terminal. J Physiol. 1973 Jan;228(2):459–497. doi: 10.1113/jphysiol.1973.sp010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C. The dependence of evoked transmitter release on external calcium ions at very low mean quantal contents. J Physiol. 1974 Jul;240(2):255–278. doi: 10.1113/jphysiol.1974.sp010609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., MACHNE X. Effect of temperature on the passive electrical properties of the muscle fibre membrane. J Physiol. 1953 May 28;120(3):431–434. doi: 10.1113/jphysiol.1953.sp004906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. J., Statham H. E. Interacting effects of temperature and extracellular calcium on the spontaneous release of transmitter at the frog neuromuscular junction. J Physiol. 1977 Jun;268(2):319–333. doi: 10.1113/jphysiol.1977.sp011859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Goto J., Kuroda H. Ultraviolet light-induced miniature end-plate potentials in frog neuromuscular junction. Experientia. 1975 Oct 15;31(10):1178–1179. doi: 10.1007/BF02326780. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W. W., Parsons R. L., Feigen G. A. Effects of temperature and drugs on mammalian motor nerve terminals. Am J Physiol. 1966 Jul;211(1):135–140. doi: 10.1152/ajplegacy.1966.211.1.135. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. The effect of temperature change upon transmitter release, facilitation and post-tetanic potentiation. J Physiol. 1971 Aug;216(3):591–609. doi: 10.1113/jphysiol.1971.sp009542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen D. W. The effect of temperature on transmission at the neuromuscular junction of the sartorius muscle of Rana pipiens. Comp Biochem Physiol A Comp Physiol. 1972 Mar;41(3):685–695. doi: 10.1016/0300-9629(72)90022-9. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Spontaneous and evoked activity of motor nerve endings in calcium Ringer. J Physiol. 1969 Aug;203(3):689–706. doi: 10.1113/jphysiol.1969.sp008887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Suppression of transmitter release at the neuromuscular junction. Proc R Soc Lond B Biol Sci. 1977 Apr;196(1125):465–469. doi: 10.1098/rspb.1977.0051. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965 Dec;181(3):656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landowne D. Sodium efflux from voltage clamped squid giant axons. J Physiol. 1977 Mar;266(1):43–68. doi: 10.1113/jphysiol.1977.sp011755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L. E., Holt J. P., Jr, Lindley B. D. Laser temperature-jump technique for relaxation studies of the ionic conductances in myelinated nerve fibers. Biophys J. 1972 Feb;12(2):157–174. doi: 10.1016/S0006-3495(72)86077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y., Alnaes E., Rahamimoff R. Is hyperosmotic neurosecretion from motor nerve endings a calcium-dependent process? Nature. 1977 May 12;267(5607):170–172. doi: 10.1038/267170a0. [DOI] [PubMed] [Google Scholar]
- Stevens C. F. A comment on Martin's relation. Biophys J. 1976 Aug;16(8):891–895. doi: 10.1016/S0006-3495(76)85739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D., Crowley W. J., Johns T. R. Effects of temperature at the neuromuscular junction. Am J Physiol. 1972 Jan;222(1):216–219. doi: 10.1152/ajplegacy.1972.222.1.216. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Erulkar S. D. Synaptic transmission and effects of temperature at the squid giant synapse. Nature. 1976 Jun 24;261(5562):720–722. doi: 10.1038/261720a0. [DOI] [PubMed] [Google Scholar]


