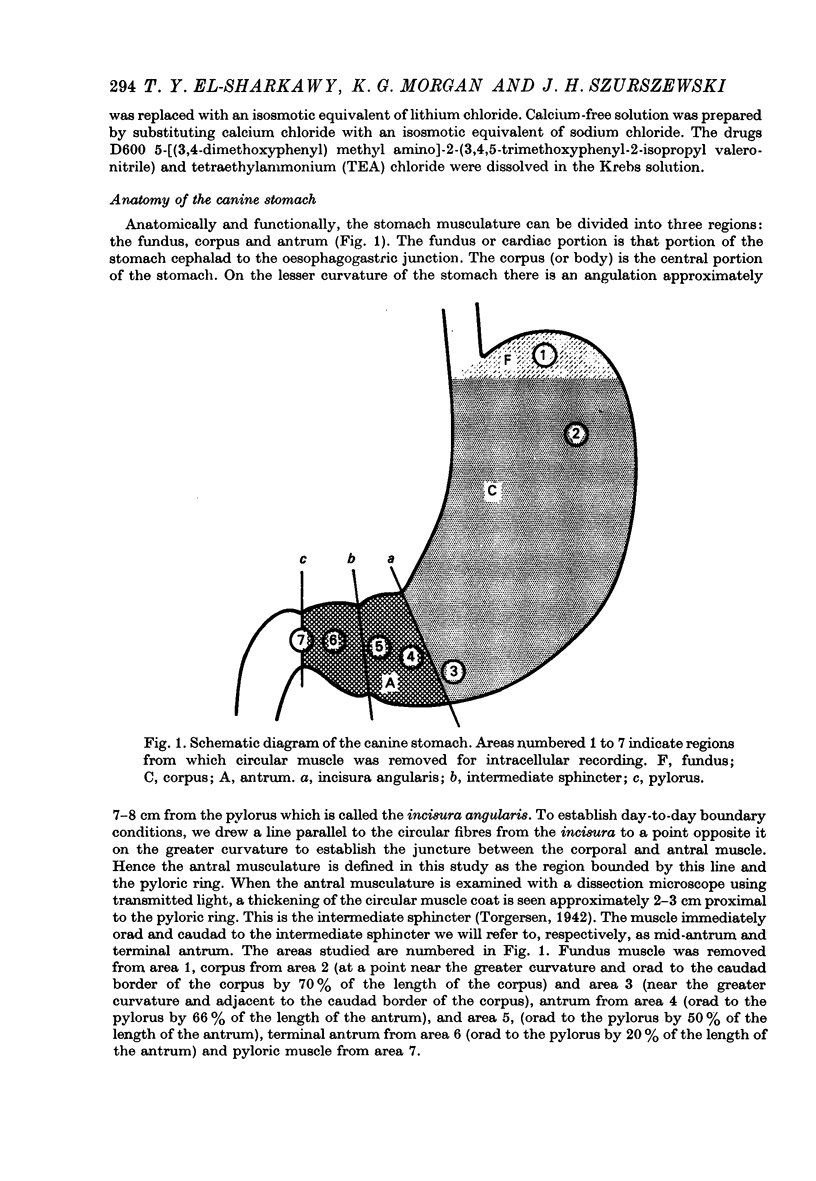
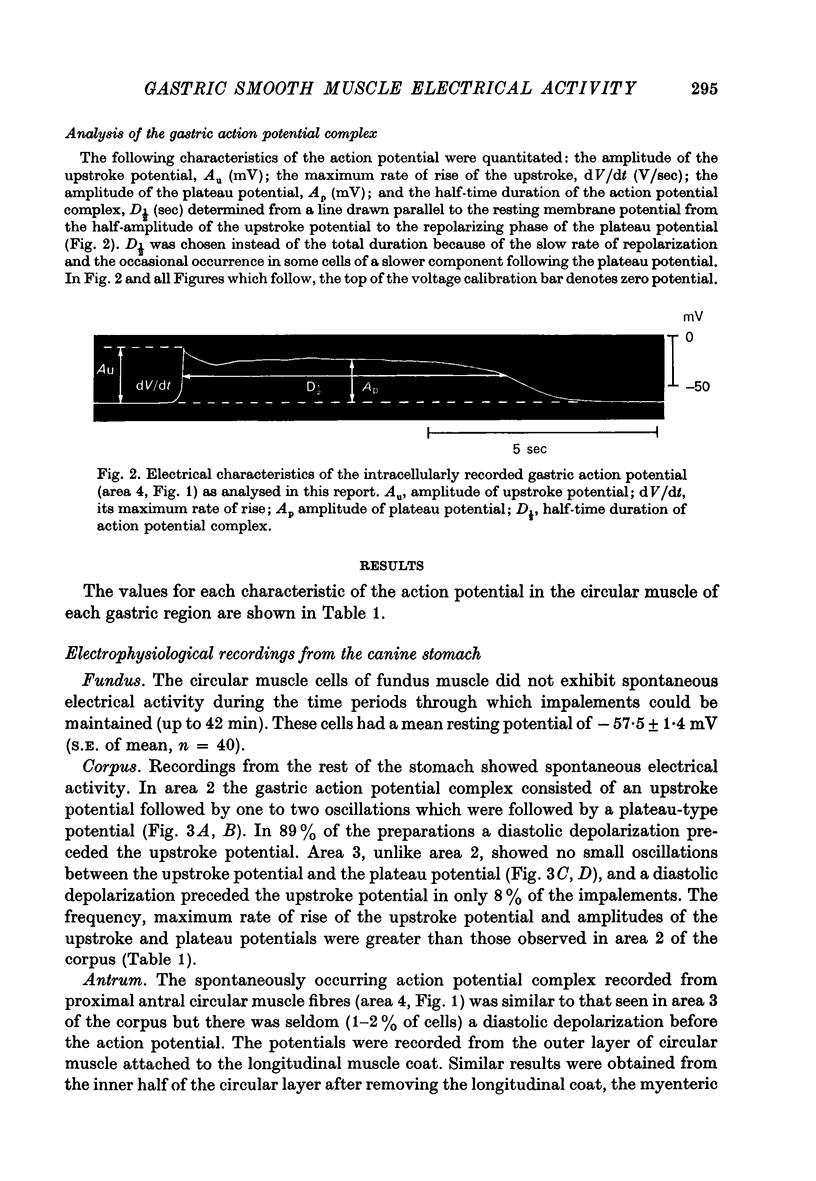
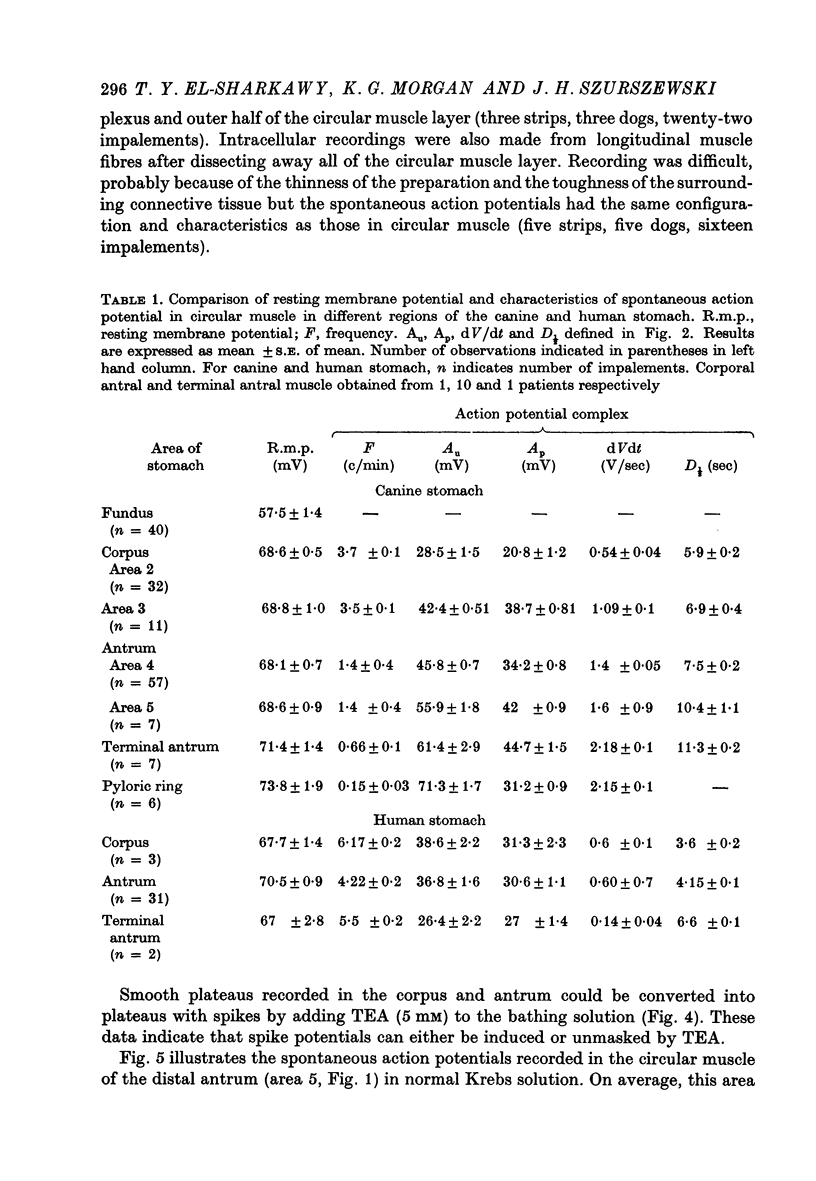
Abstract
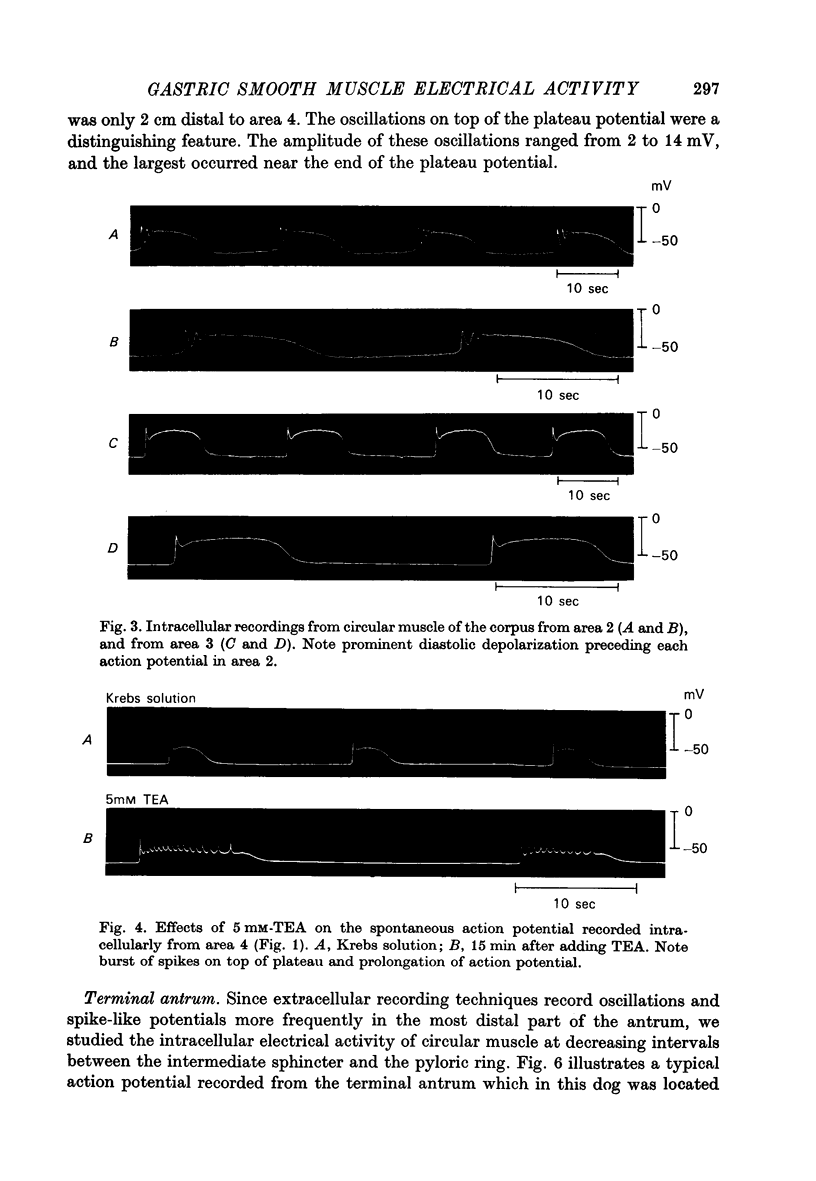
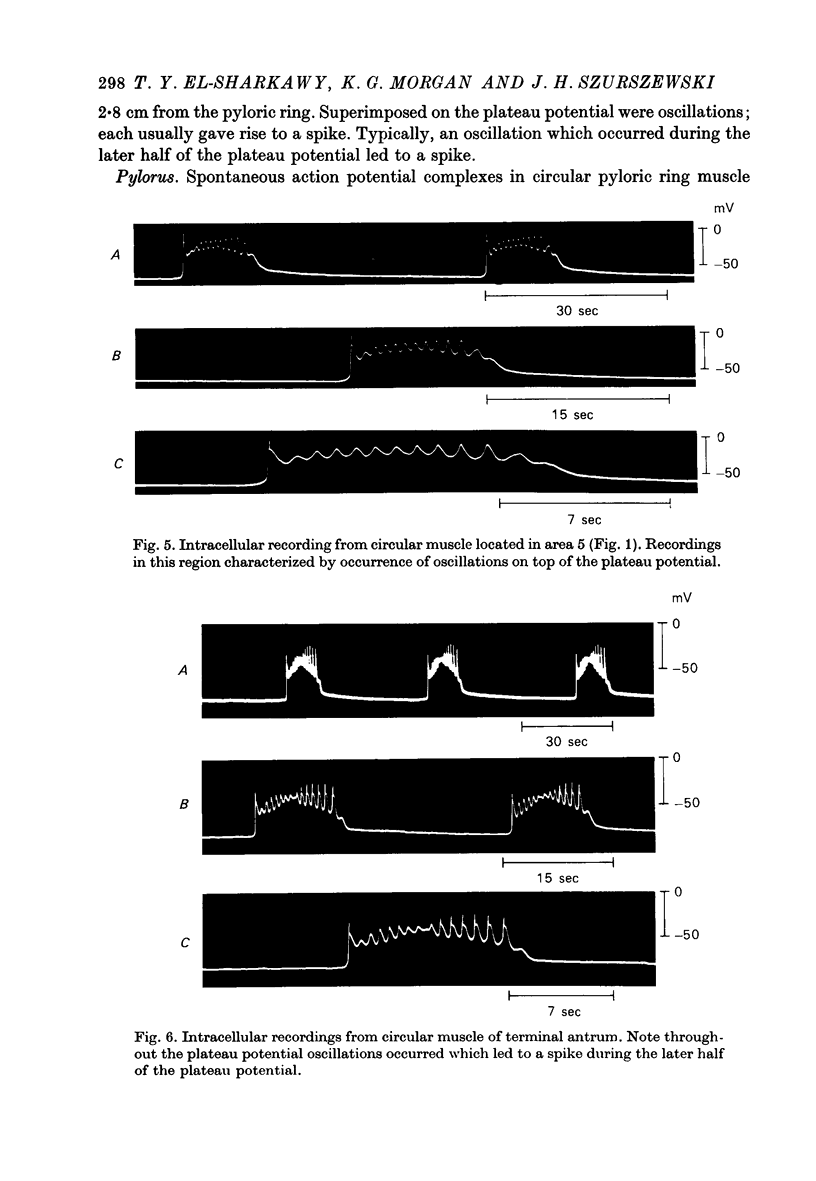
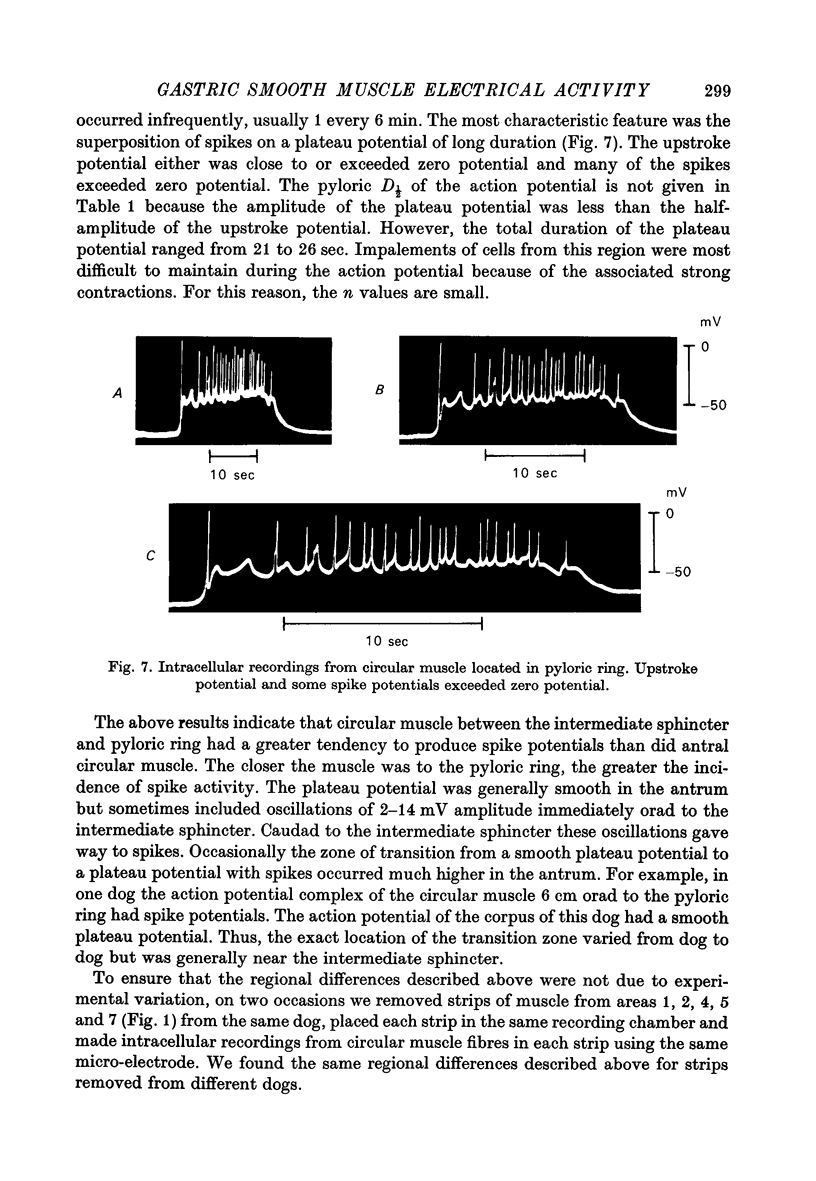
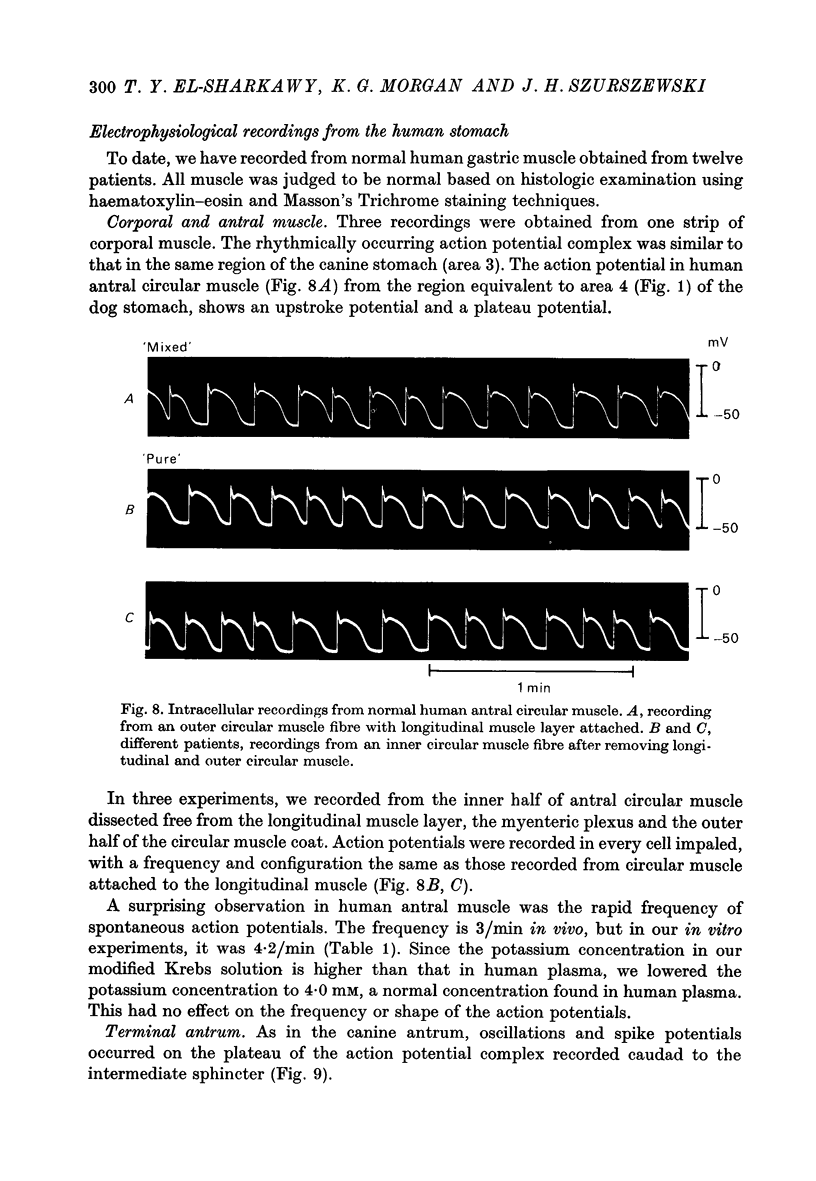
1. Intracellular recordings were obtained from circular smooth muscle fibres of the canine fundus, corpus, antrum and pylorus as well as from the human corpus and antrum. 2. In the canine stomach, all regions of the stomach except the fundus exhibited spontaneous action potentials. 3. The spontaneous action potential consisted of an upstroke potential and a plateau potential. 4. There were regional differences in the configuration of the plateau potential. Corporal and antral smooth muscle did not normally spike during the plateau potential whereas terminal antral and pyloric muscle usually showed spikes on top of the plateau potential. Near the intermediate sphincter, there was a zone of transition in which oscillations in potential of variable amplitude were superimposed on the plateau potential. 5. The configuration of the action potential of the human stomach was similar to the configuration of the canine action potential when the same region of the stomach was compared. 6. The ionic dependence of the plateau potential was studied in canine stomach in an area where neither oscillations nor spikes occurred. 7. In clacium-free solution, all spontaneous activity stopped. D600 selectively suppressed the size of the plateau potential. 8. Sodium-deficient solution reduced the size of the plateau potential. 9. These results suggest that both calcium and sodium may be involved as current carriers in the generation of the plateau potential.
Full text
PDF


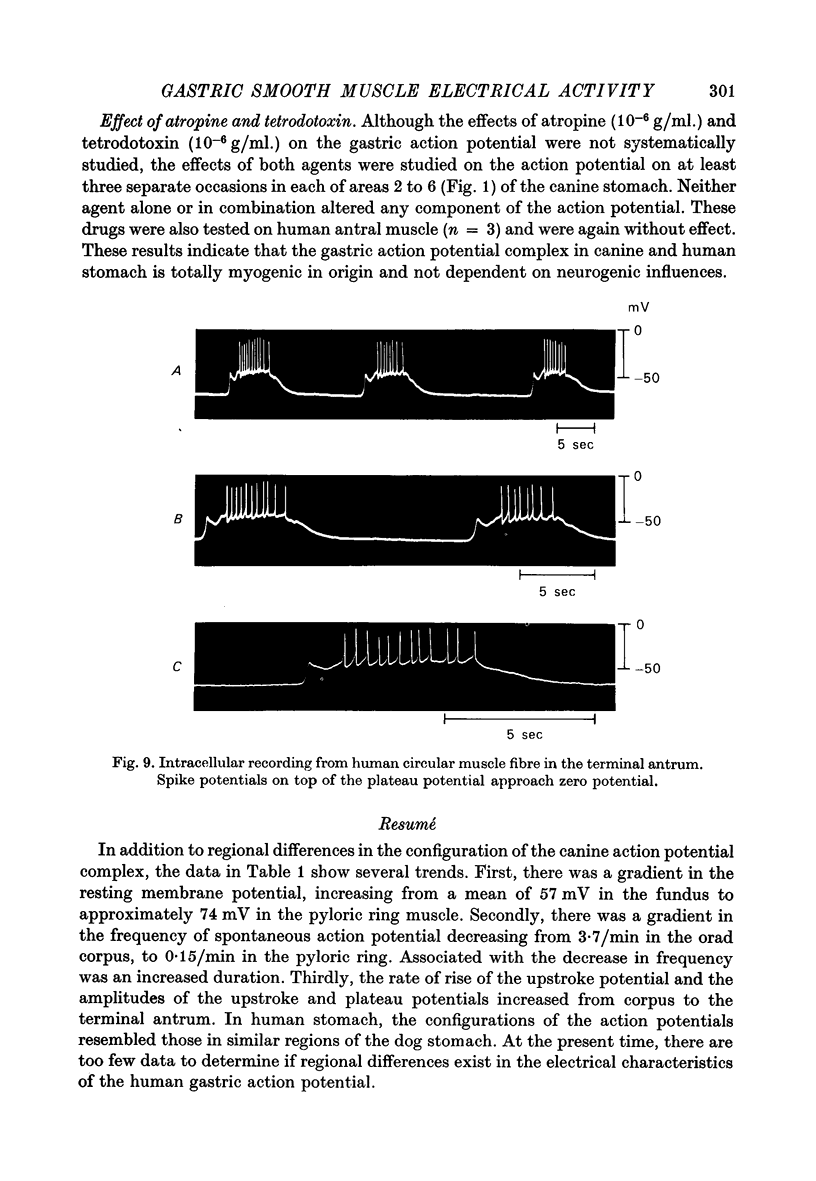
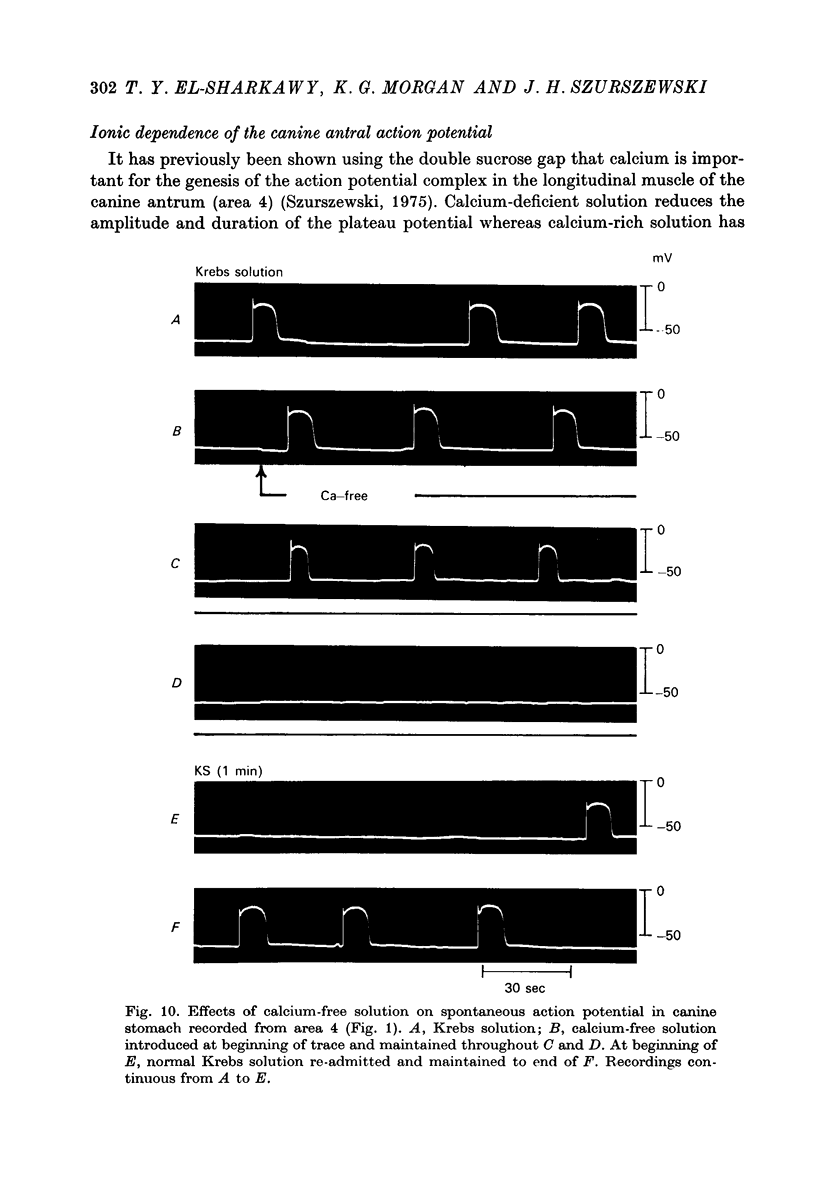
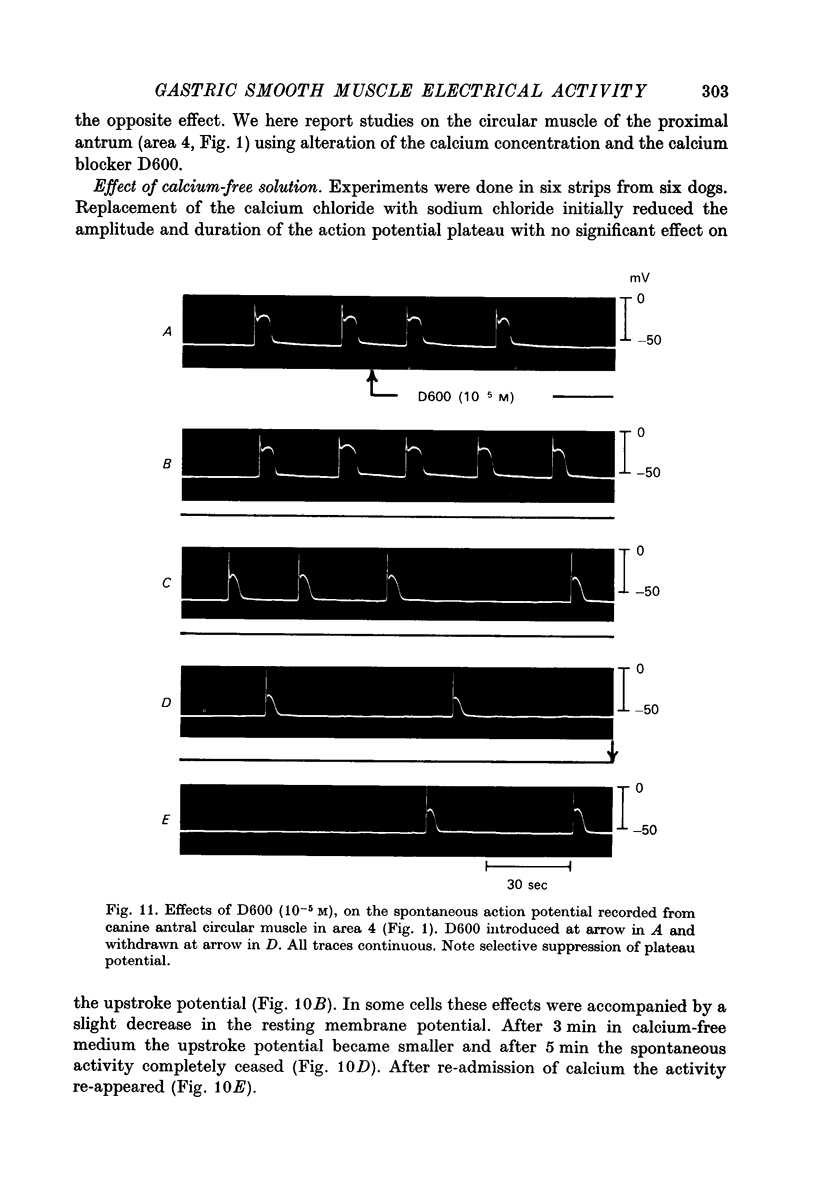
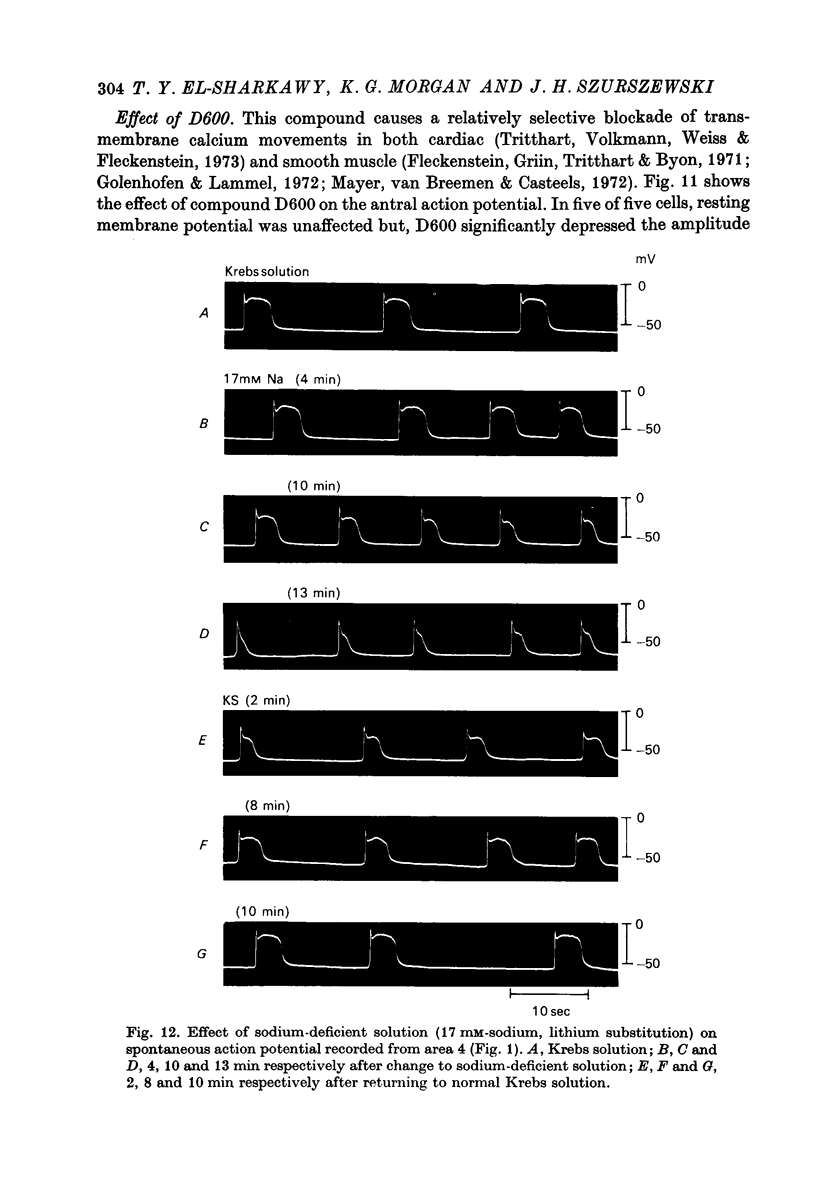



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlson H. C., Code C. F., Nelson R. A. Motor action of the canine gastroduodenal junction: a cineradiographic, pressure, and electric study. Am J Dig Dis. 1966 Feb;11(2):155–172. doi: 10.1007/BF02239239. [DOI] [PubMed] [Google Scholar]
- Daniel E. E. Electrical and contractile responses of the pyloric region to adrenergic and cholinergic drugs. Can J Physiol Pharmacol. 1966 Nov;44(6):951–979. doi: 10.1139/y66-117. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A., Grün G., Tritthart H., Byon K. Uterus-Relaxation durch hochaktive Ca plus,plus-antagonistische Hemmstoffe der elektro-mechanischen Koppelung wie Isoptin (Verapamil, Iproveratril), Substanz D 600 und Segontin (Prenylamin). Versuche am isolierten Uterus virgineller Ratten. Klin Wochenschr. 1971 Jan;49(1):32–41. doi: 10.1007/BF01494064. [DOI] [PubMed] [Google Scholar]
- Golenhofen K., Lammel E. Selective suppression of some components of spontaneous activity in various types of smooth muscle by iproveratril (Verapamil). Pflugers Arch. 1972;331(3):233–243. [PubMed] [Google Scholar]
- Hinder R. A., Kelly K. A. Human gastric pacesetter potential. Site of origin, spread, and response to gastric transection and proximal gastric vagotomy. Am J Surg. 1977 Jan;133(1):29–33. doi: 10.1016/0002-9610(77)90187-8. [DOI] [PubMed] [Google Scholar]
- KAO C. Y., NISHIYAMA A. OVARIAN HORMONES AND RESTING POTENTIAL OF RABBIT UTERINE SMOOTH MUSCLE. Am J Physiol. 1964 Oct;207:793–799. doi: 10.1152/ajplegacy.1964.207.4.793. [DOI] [PubMed] [Google Scholar]
- Kelly K. A., Code C. F. Canine gastric pacemaker. Am J Physiol. 1971 Jan;220(1):112–118. doi: 10.1152/ajplegacy.1971.220.1.112. [DOI] [PubMed] [Google Scholar]
- Kelly K. A., Code C. F., Elveback L. R. Patterns of canine gastric electrical activity. Am J Physiol. 1969 Aug;217(2):461–470. doi: 10.1152/ajplegacy.1969.217.2.461. [DOI] [PubMed] [Google Scholar]
- Kelly K. A. Gastric motility after gastric operations. Surg Annu. 1974;6:103–123. [PubMed] [Google Scholar]
- Mayer C. J., van Breemen C., Casteels T. The action of lanthanum and D600 on the calcium exchange in the smooth muscle cells of the guinea-pig Taenia coli. Pflugers Arch. 1972;337(4):333–350. doi: 10.1007/BF00586650. [DOI] [PubMed] [Google Scholar]
- Papasova M. P., Nagai T., Prosser C. L. Two-component slow waves in smooth muscle of cat stomach. Am J Physiol. 1968 Apr;214(4):695–702. doi: 10.1152/ajplegacy.1968.214.4.695. [DOI] [PubMed] [Google Scholar]
- SUGAWARA K. AN ELECTROMYOGRAPHIC STUDY ON THE MOTILITY OF CANINE STOMACH AFTER TRANSECTION AND END-TO-END ANASTOMOSIS. Tohoku J Exp Med. 1964 Nov 25;84:113–124. doi: 10.1620/tjem.84.113. [DOI] [PubMed] [Google Scholar]
- Szurszewski J. H. Mechanism of action of pentagastrin and acetylcholine on the longitudinal muscle of the canine antrum. J Physiol. 1975 Nov;252(2):335–361. doi: 10.1113/jphysiol.1975.sp011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritthart H., Volkmann R., Weiss R., Fleckenstein A. Calcium-mediated action potentials in mammalian myocardium. Alteration of membrane response as induced by changes of Cae or by promoters and inhibitors of transmembrane Ca inflow. Naunyn Schmiedebergs Arch Pharmacol. 1973;280(3):239–252. doi: 10.1007/BF00501349. [DOI] [PubMed] [Google Scholar]
- Weber J., Jr, Koatsu S. Pacemaker localization and electrical conduction patterns in the canine stomach. Gastroenterology. 1970 Nov;59(5):717–726. [PubMed] [Google Scholar]