Abstract
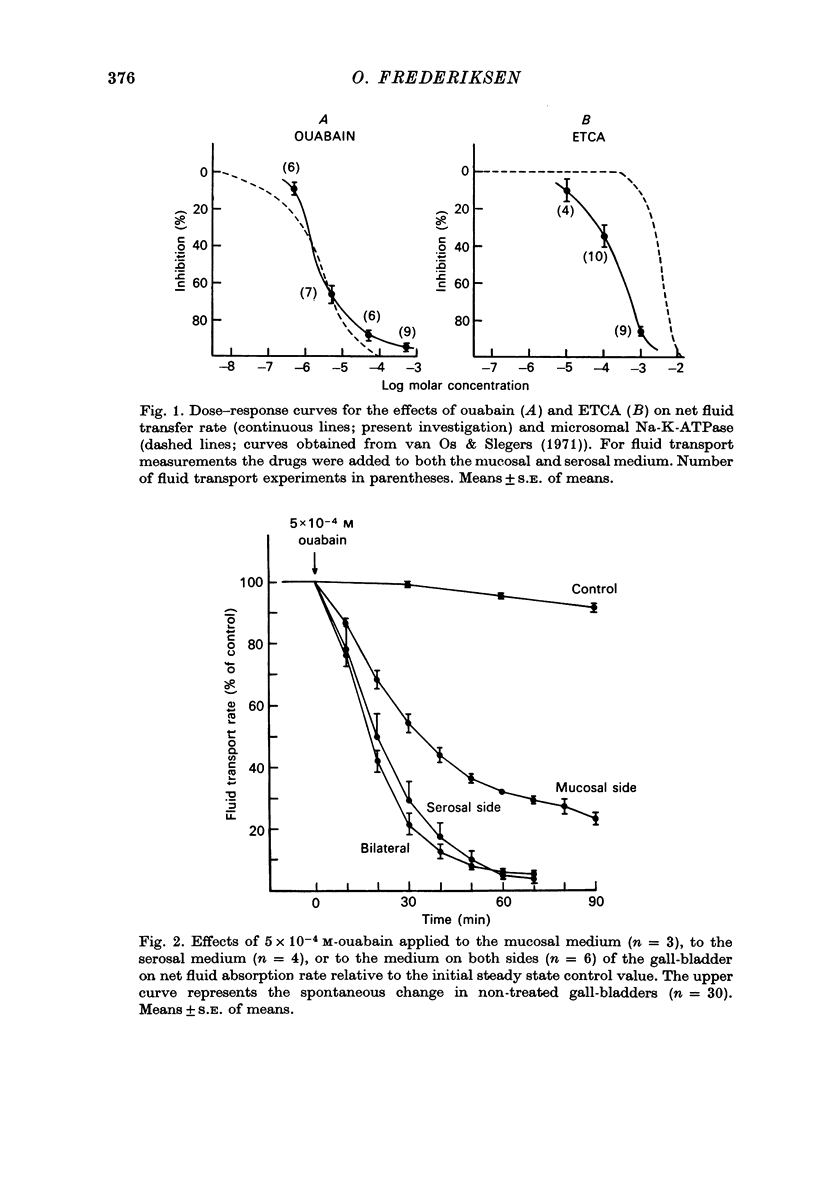
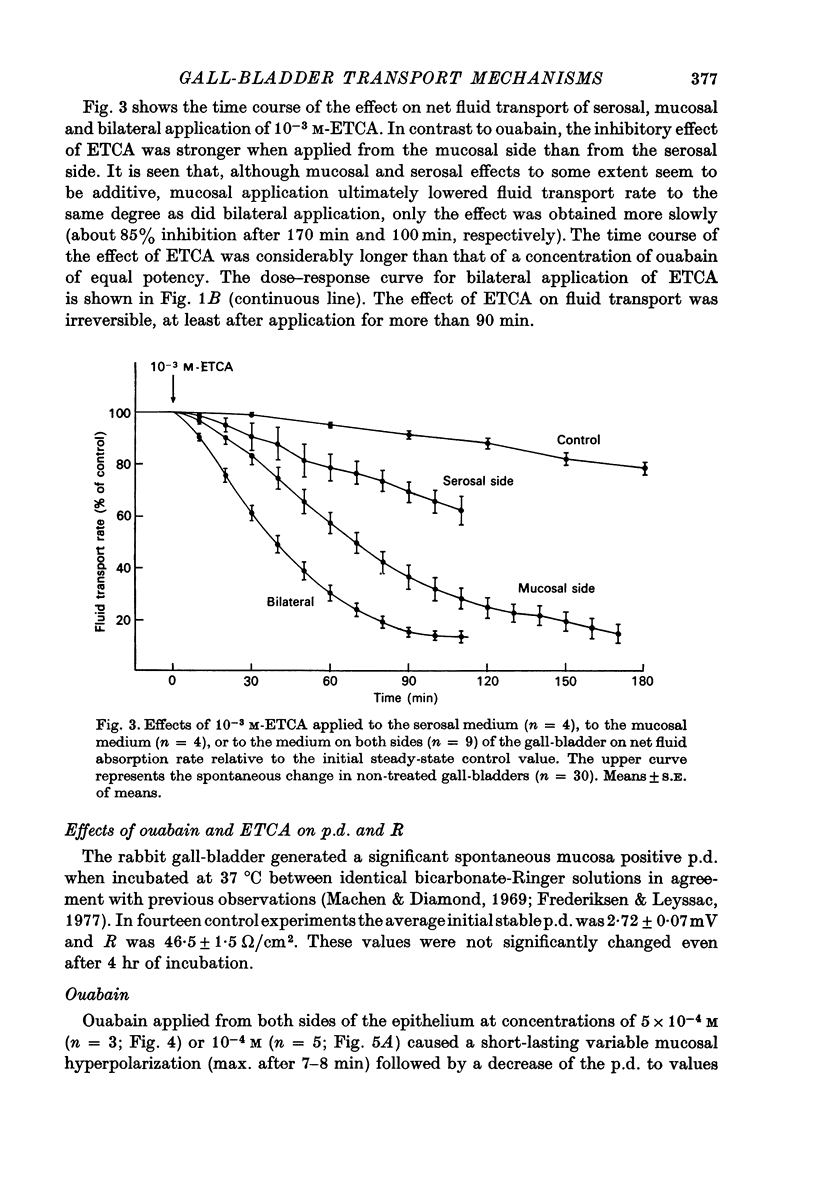
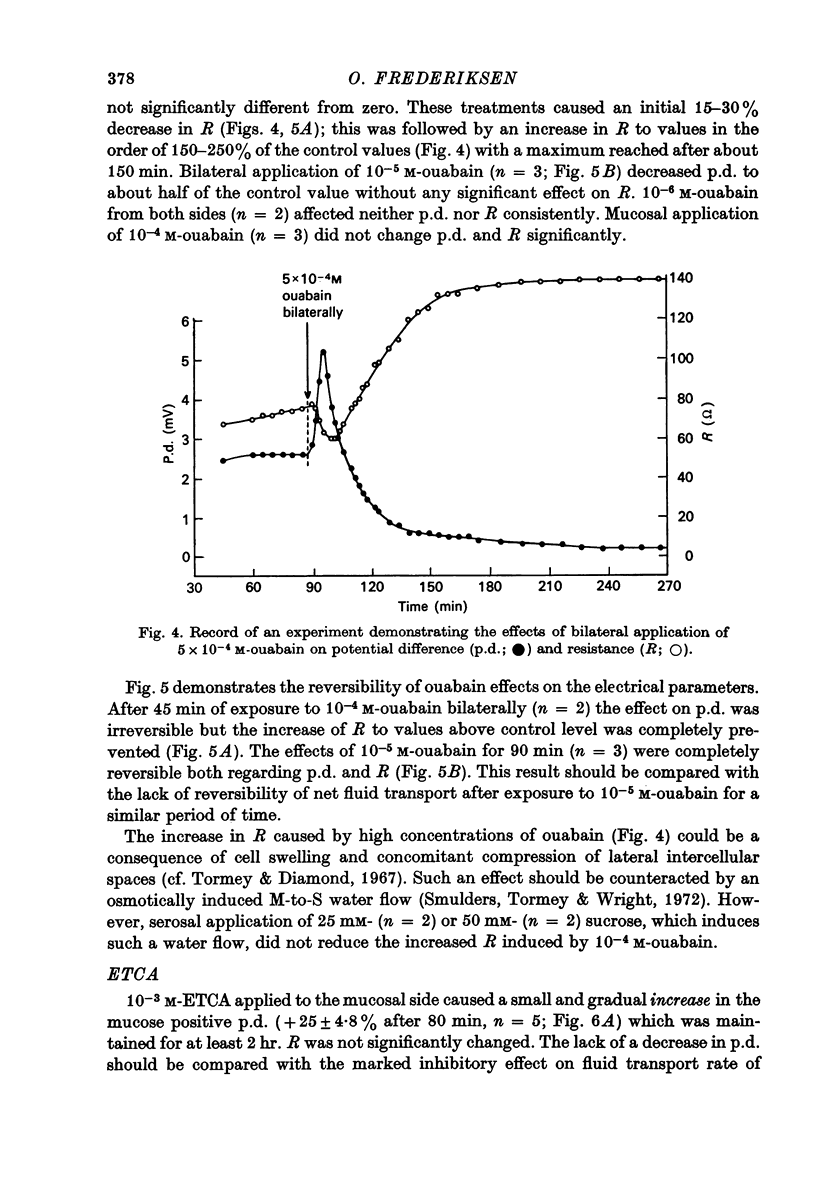
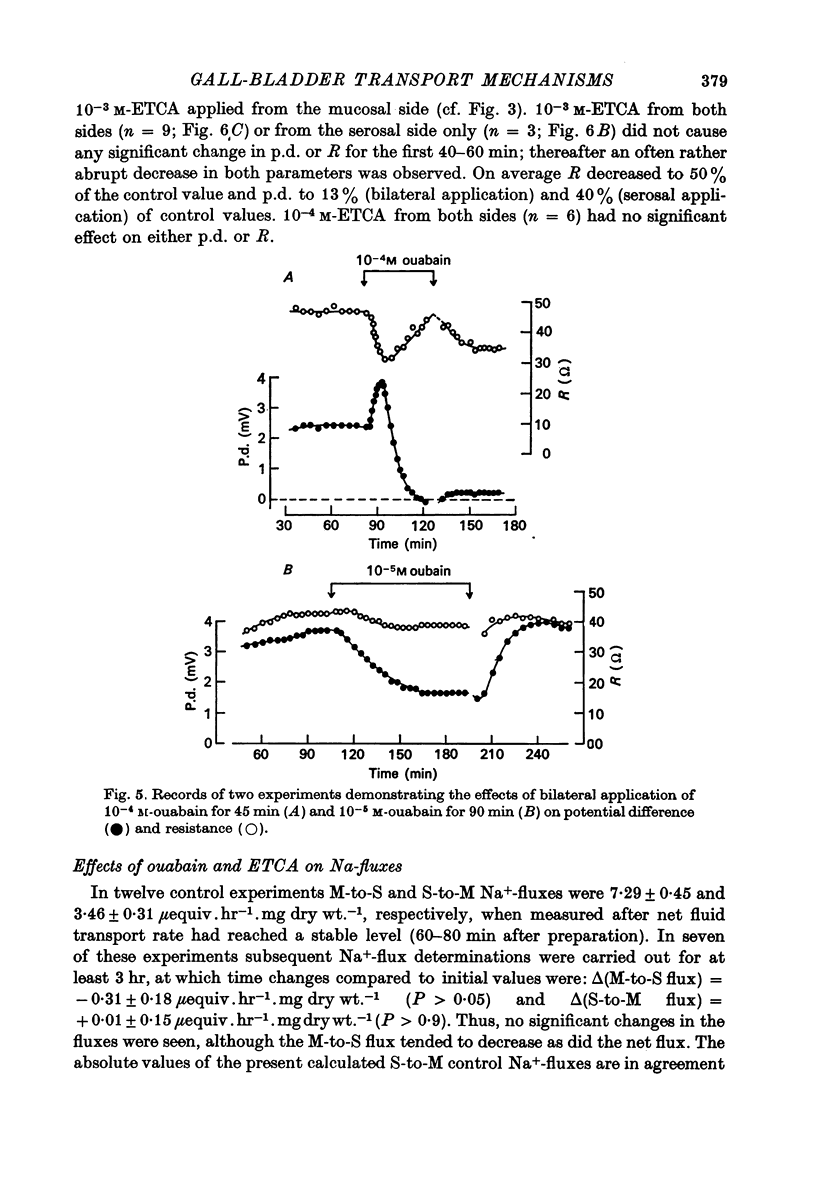
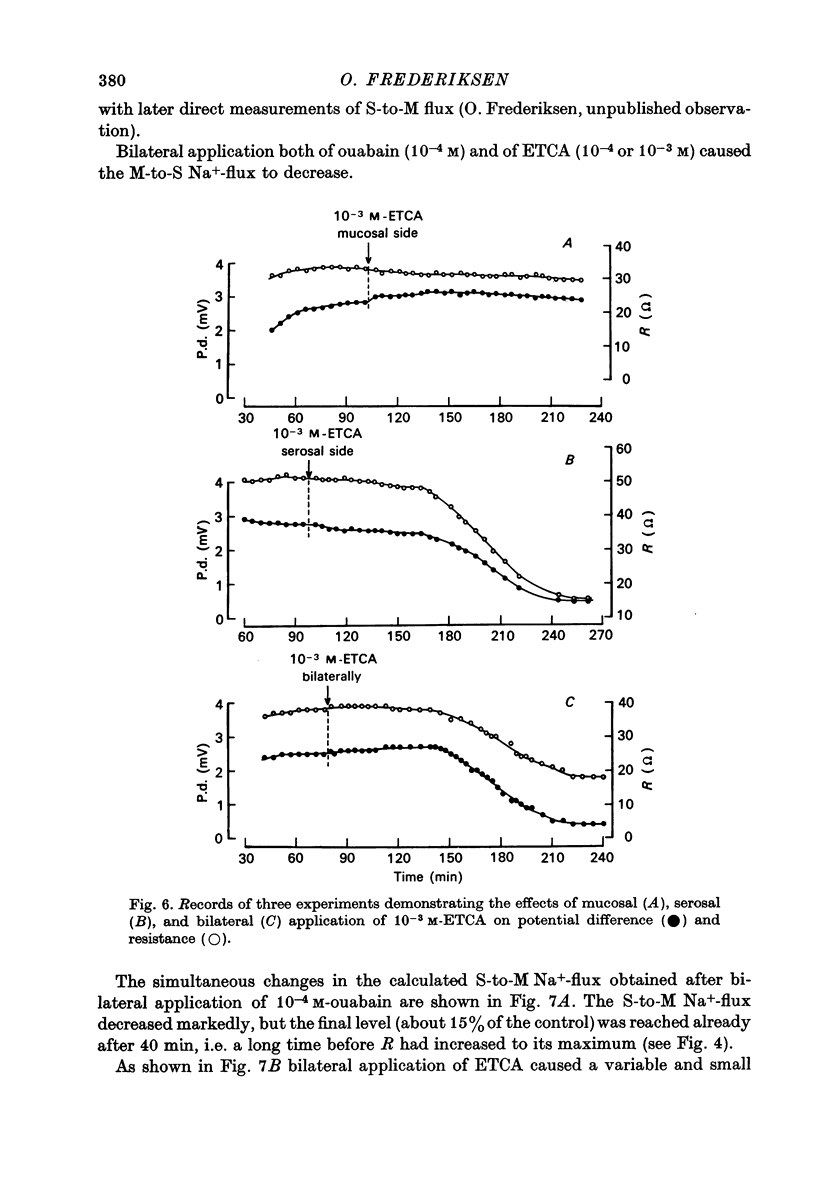
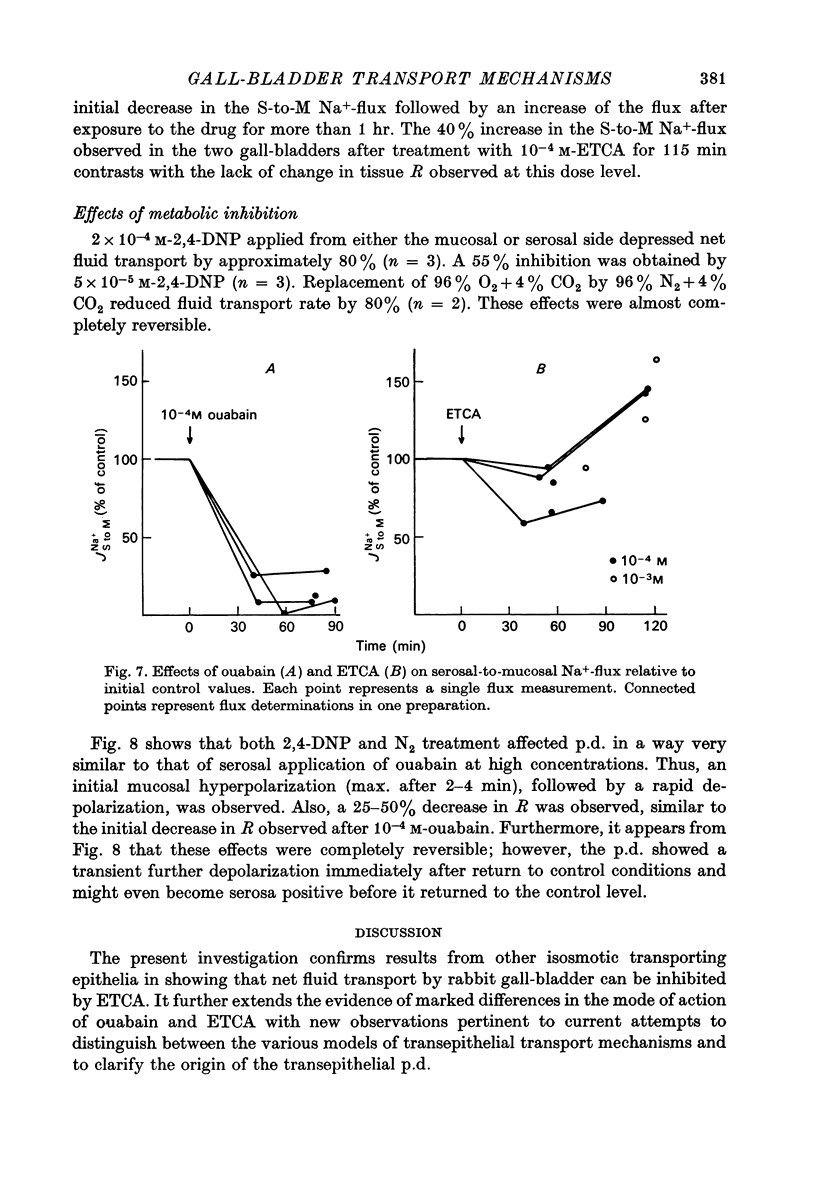
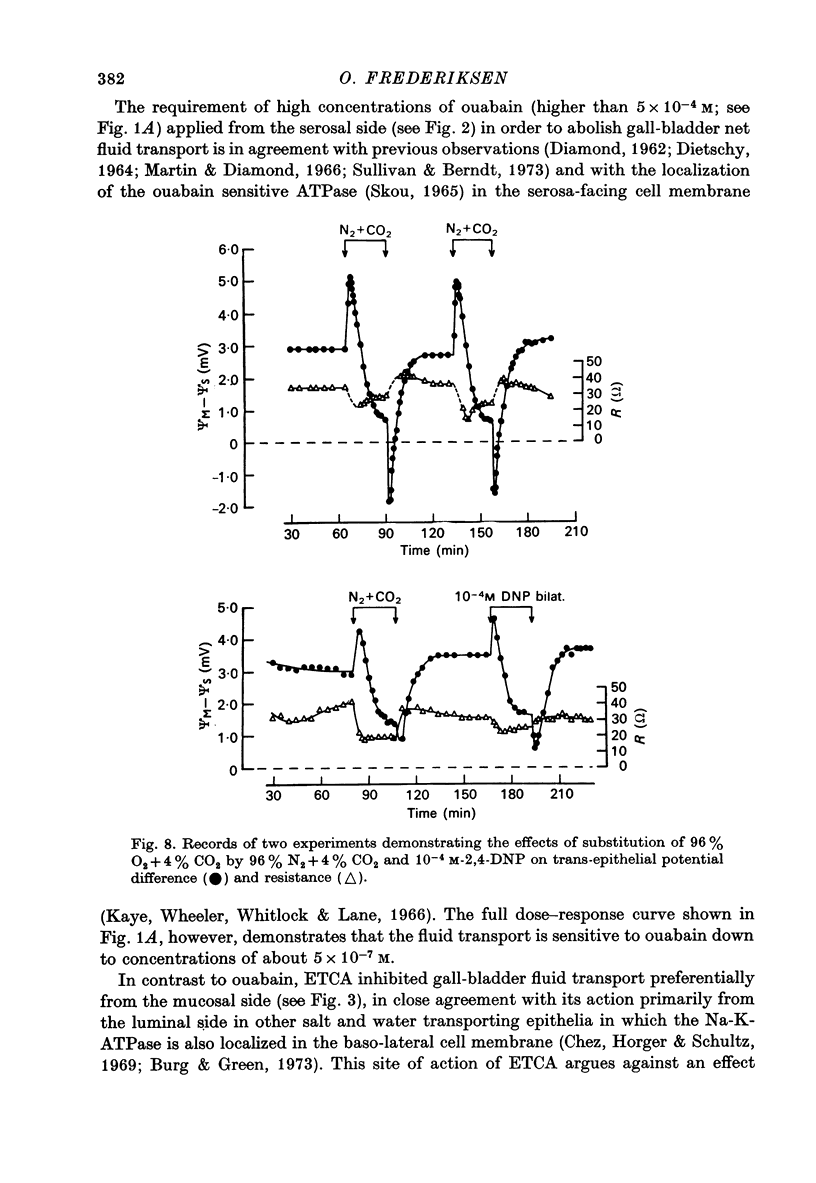
1. Net fluid transport rate, transepithelial p.d. and resistance, and unidirectional Na+-fluxes were measured in rabbit gall-bladder preparations exposed on both sides to bicarbonate-Ringer solution in vitro. 2. Both ouabain and ethacrynic acid (ETCA) caused dose-dependent decreases of net fluid transport rate; ouabain inhibited fluid transport predominantly from the serosal side, whereas the inhibitory effect of ETCA was elicited mainly from the mucosal (luminal) side. Applied bilaterally, the ID50 for ouabain was 2.5 X 10(-6) M, and for ETCA 2.3 X 10(-4) M. After maximal inhibition at each concentration level of the two inhibitors fluid transport could not be reversed. 3. 2,4-Dinitrophenol (2,4-DNP) (2 X 10(-4) M) or substitution of O2 by N2 caused an 80% reversible decrease of net fluid transport. 4. The spontaneous p.d. across the rabbit gall-bladder was about 2.7 mV, mucosal side positive. 2,4-DNP, N2 and serosal application of ouabain depressed the p.d. after an initial hyperpolarization. This decrease was reversible during recovery from 2,4-DNP and N2, but irreversible after removal of ouabain at concentrations greater than or equal to 10(-4) M. Mucosal application of ETCA (10(-3) M) caused no decrease in p.d., which actually increased slightly. 5. Calculated passive serosal-to-mucosal Na+-fluxes changed in the same direction as did changes in conductance. 6. It is concluded that ETCA does not interfere primarily with the Na-K-ATPase or cellular oxidative metabolism. The data support the proposal that the pump responsible for isosmotic transepithelial fluid transfer is located in the luminal end of the cells. This pump is ETCA-sensitive. The ATPase-dependent Na-K pump, which can be inhibited by ouabain, is localized in the serosa-facing cell membrane. The data suggest that the inhibition of net fluid transport by ouabain is indirect and mediated by changes in intracellular ion concentrations. 7. The results support the concept that the transepithelial fluid transport mechanism is electroneutral, and suggest that the mucosa positive transepithelial p.d. is due to differences in electromotive forces arising from ion (mainly K+) diffusion across the mucosal and serosal cell membranes.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burg M., Green N. Effect of ethacrynic acid on the thick ascending limb of Henle's loop. Kidney Int. 1973 Nov;4(5):301–308. doi: 10.1038/ki.1973.121. [DOI] [PubMed] [Google Scholar]
- DIAMOND J. M. THE MECHANISM OF ISOTONIC WATER TRANSPORT. J Gen Physiol. 1964 Sep;48:15–42. doi: 10.1085/jgp.48.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J. M. The reabsorptive function of the gall-bladder. J Physiol. 1962 May;161:442–473. doi: 10.1113/jphysiol.1962.sp006898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIETSCHY J. M. WATER AND SOLUTE MOVEMENT ACROSS THE WALL OF THE EVERTED RABBIT GALL BLADDER. Gastroenterology. 1964 Oct;47:395–408. [PubMed] [Google Scholar]
- Diamond J. M., Bossert W. H. Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J Gen Physiol. 1967 Sep;50(8):2061–2083. doi: 10.1085/jgp.50.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel H., Ehrich J., De Santo N. G., Doerken U. Plasma membranes of the kidney. 3. Influence of diuretics on ATPase activity. Pflugers Arch. 1972;335(3):224–234. doi: 10.1007/BF00592159. [DOI] [PubMed] [Google Scholar]
- Epstein R. W. The effects of ethacrynic acid on active transport of sugars and ions and on other metabolic processes in rabbit kidney cortex. Biochim Biophys Acta. 1972 Jul 3;274(1):128–139. doi: 10.1016/0005-2736(72)90288-x. [DOI] [PubMed] [Google Scholar]
- Frederiksen O., Leyssac P. P. Effects of cytochalasin B and dimethylsulphoxide on isosmotic fluid transport by rabbit gall-bladder in vitro. J Physiol. 1977 Feb;265(1):103–118. doi: 10.1113/jphysiol.1977.sp011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen O., Leyssac P. P. Transcellular transport of isosmotic volumes by the rabbit gall-bladder in vitro. J Physiol. 1969 Mar;201(1):201–224. doi: 10.1113/jphysiol.1969.sp008751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frömter E., Gessner K. Active transport potentials, membrane diffusion potentials and streaming potentials across rat kidney proximal tubule. Pflugers Arch. 1974;351(1):85–98. doi: 10.1007/BF00603513. [DOI] [PubMed] [Google Scholar]
- Frömter E. The route of passive ion movement through the epithelium of Necturus gallbladder. J Membr Biol. 1972;8(3):259–301. doi: 10.1007/BF01868106. [DOI] [PubMed] [Google Scholar]
- Giebisch G., Sullivan L. P., Whittembury G. Relationship between tubular net sodium reabsorption and peritubular potassium uptake in the perfused Necturus kidney. J Physiol. 1973 Apr;230(1):51–74. doi: 10.1113/jphysiol.1973.sp010174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hénin S., Cremaschi D. Transcellular ion route in rabbit gallbladder. Electric properties of the epithelial cells. Pflugers Arch. 1975;355(2):125–139. doi: 10.1007/BF00581828. [DOI] [PubMed] [Google Scholar]
- Inagaki C., Martinez-Maldonado M., Schwartz A. Some in vivo and in vitro effects of ethacrynic acid on renal Na+,K+ -ATPase. Arch Biochem Biophys. 1973 Sep;158(1):421–434. doi: 10.1016/0003-9861(73)90639-5. [DOI] [PubMed] [Google Scholar]
- KLEINZELLER A., KNOTKOVA A. THE EFFECT OF OUABAIN ON THE ELECTROLYTE AND WATER TRANSPORT IN KIDNEY CORTEX AND LIVER SLICES. J Physiol. 1964 Dec;175:172–192. doi: 10.1113/jphysiol.1964.sp007510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORN R., CAFRUNY E. J. EFFECTS OF ETHACRYNIC ACID ON RENAL PROTEIN-BOUND SULFHYDRYL GROUPS. J Pharmacol Exp Ther. 1965 Jun;148:367–372. [PubMed] [Google Scholar]
- Kaye G. I., Wheeler H. O., Whitlock R. T., Lane N. Fluid transport in the rabbit gallbladder. A combined physiological and electron microscopic study. J Cell Biol. 1966 Aug;30(2):237–268. doi: 10.1083/jcb.30.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyssac P. P., Bukhave K., Frederiksen O. Inhibitory effect of prostaglandins on isosmotic fluid transport by rabbit gall-bladder in vitro, and its modification by blocade of endogenous PGE-Biosynthesis with indomethacin. Acta Physiol Scand. 1974 Dec;92(4):496–507. doi: 10.1111/j.1748-1716.1974.tb05771.x. [DOI] [PubMed] [Google Scholar]
- Lutz M. D., Cardinal J., Burg M. B. Electrical resistance of renal proximal tubule perfused in vitro. Am J Physiol. 1973 Sep;225(3):729–734. doi: 10.1152/ajplegacy.1973.225.3.729. [DOI] [PubMed] [Google Scholar]
- Machen T. E., Erlij D., Wooding F. B. Permeable junctional complexes. The movement of lanthanum across rabbit gallbladder and intestine. J Cell Biol. 1972 Aug;54(2):302–312. doi: 10.1083/jcb.54.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight A. D. Water and electrolyte contents of rat renal cortical slices incubated in potassium-free media and media containing ouabain. Biochim Biophys Acta. 1968 Mar 1;150(2):263–270. doi: 10.1016/0005-2736(68)90169-7. [DOI] [PubMed] [Google Scholar]
- Martin D. W., Diamond J. M. Energetics of coupled active transport of sodium and chloride. J Gen Physiol. 1966 Nov;50(2):295–315. doi: 10.1085/jgp.50.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude D. L. Mechanism of salt transport and some permeability properties of rat proximal tubule. Am J Physiol. 1970 Jun;218(6):1590–1595. doi: 10.1152/ajplegacy.1970.218.6.1590. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Electrical properties of the cellular transepithelial pathway in Necturus gallbladder. I. Circuit analysis and steady-state effects of mucosal solution ionic substitutions. J Membr Biol. 1975 Dec 4;25(1-2):115–139. doi: 10.1007/BF01868571. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Electrical properties of the cellular transepithelial pathway in Necturus gallbladder. II. Ionic permeability of the apical cell membrane. J Membr Biol. 1975 Dec 4;25(1-2):141–161. doi: 10.1007/BF01868572. [DOI] [PubMed] [Google Scholar]
- Rorive G., Kleinzeller A. The effect of ATP and Ca 2+ on the cell volume in isolated kidney tubules. Biochim Biophys Acta. 1972 Jul 3;274(1):226–239. doi: 10.1016/0005-2736(72)90296-9. [DOI] [PubMed] [Google Scholar]
- Rorive G., Nielsen R., Kleinzeller A. Effect of pH on the water and electrolyte content of renal cells. Biochim Biophys Acta. 1972 May 9;266(2):376–396. doi: 10.1016/0005-2736(72)90095-8. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Schultz S. G. Electrical potential differences and electromotive forces in epithelial tissues. J Gen Physiol. 1972 Jun;59(6):794–798. doi: 10.1085/jgp.59.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan B., Berndt W. O. Transport by isolated rabbit gallbladders in bicarbonate-buffered solutions. Am J Physiol. 1973 Oct;225(4):845–848. doi: 10.1152/ajplegacy.1973.225.4.845. [DOI] [PubMed] [Google Scholar]
- Tormey J. M., Diamond J. M. The ultrastructural route of fluid transport in rabbit gall bladder. J Gen Physiol. 1967 Sep;50(8):2031–2060. doi: 10.1085/jgp.50.8.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Os C. H., Slegers J. F. Characteristics of Naplus-Kplus-stimulated ATPase in rabbit gall bladder epithelium. Pflugers Arch. 1970;319(1):49–56. doi: 10.1007/BF00586427. [DOI] [PubMed] [Google Scholar]
- Whittembury G., Fishman J. Relation between cell Na extrusion and transtubular absorption in the perfused toad kidney: the effect of K, ouabain and ethacrynic acid. Pflugers Arch. 1969;307(3):138–153. doi: 10.1007/BF00592080. [DOI] [PubMed] [Google Scholar]
- Whittembury G., Proverbio F. Two modes of Na extrusion in cells from guinea pig kidney cortex slices. Pflugers Arch. 1970;316(1):1–25. doi: 10.1007/BF00587893. [DOI] [PubMed] [Google Scholar]
- van Os C. H., Slegers J. F. Correlation between (Na + -K + )-activated ATPase activities and the rate of isotonic fluid transport of gallbladder epithelium. Biochim Biophys Acta. 1971 Jul 6;241(1):89–96. doi: 10.1016/0005-2736(71)90306-3. [DOI] [PubMed] [Google Scholar]
- van Os C. H., Slegers J. F. The electrical potential profile of gallbladder epithelium. J Membr Biol. 1975 Dec 4;24(3-4):341–363. doi: 10.1007/BF01868631. [DOI] [PubMed] [Google Scholar]


