Abstract
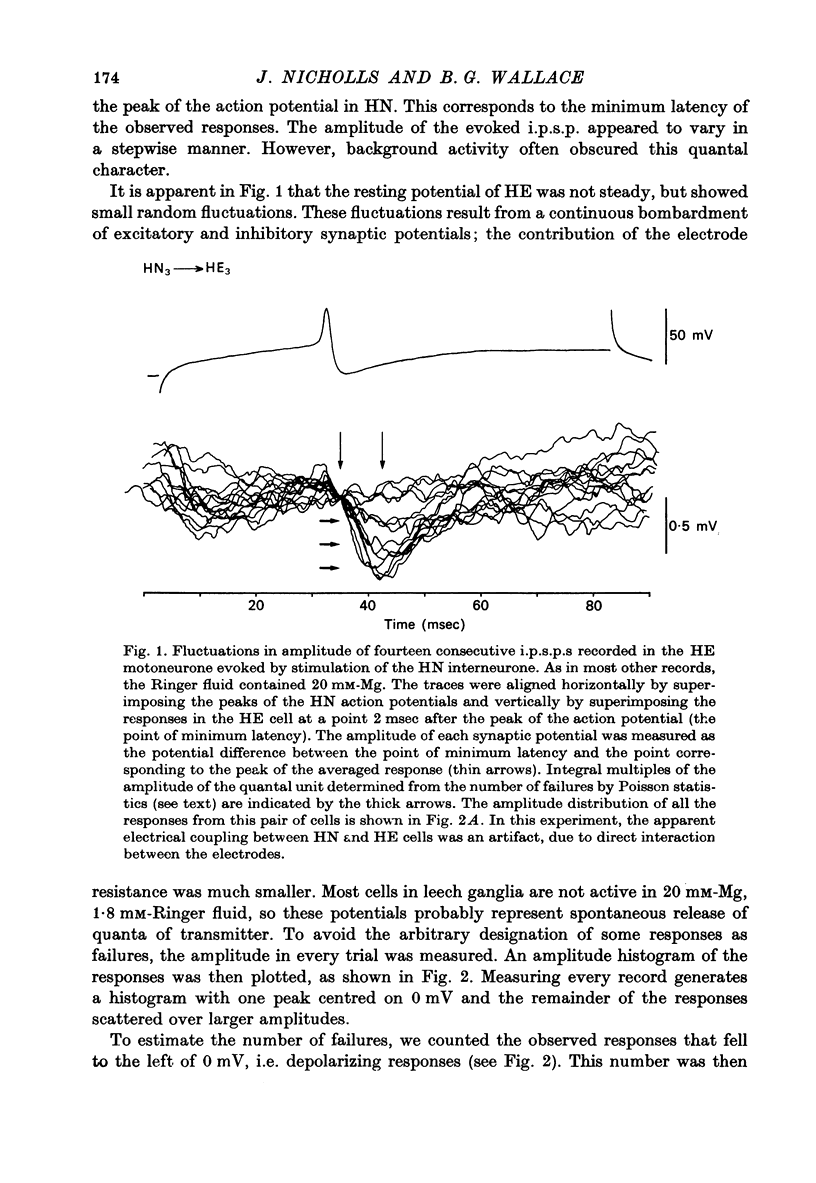
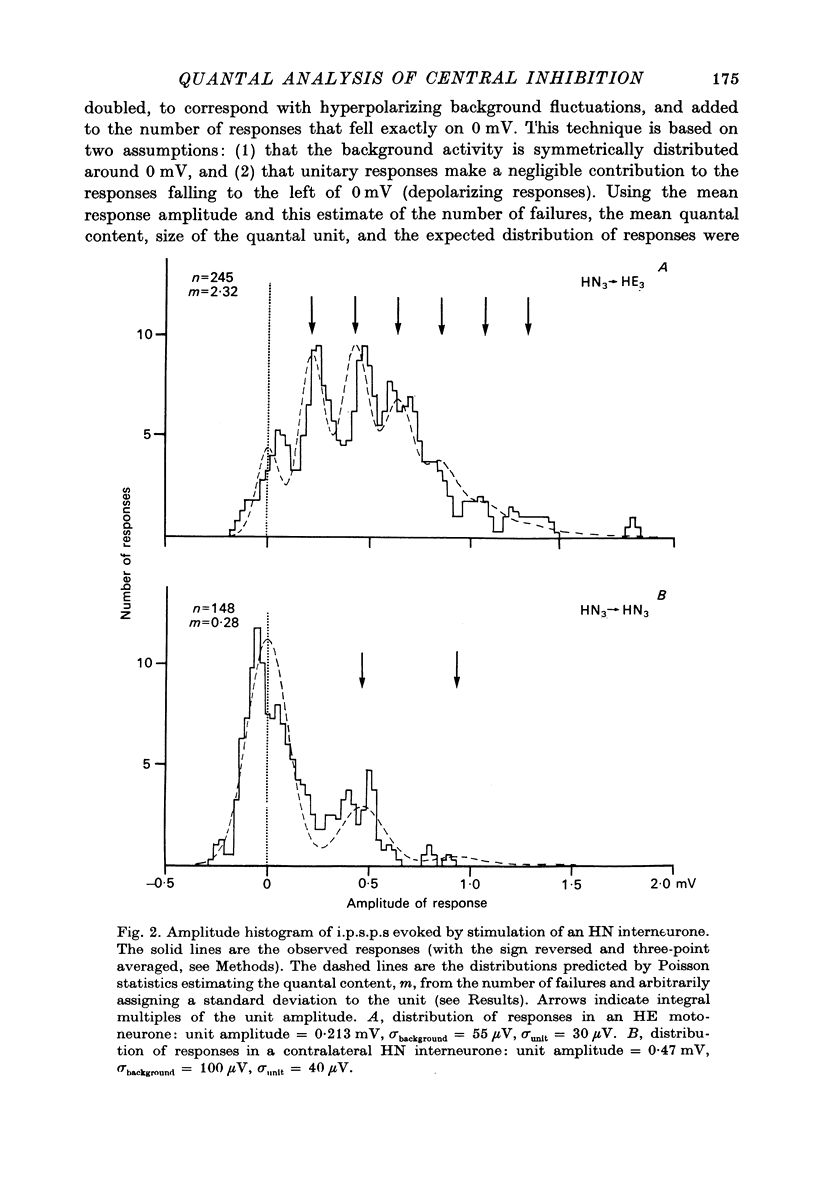
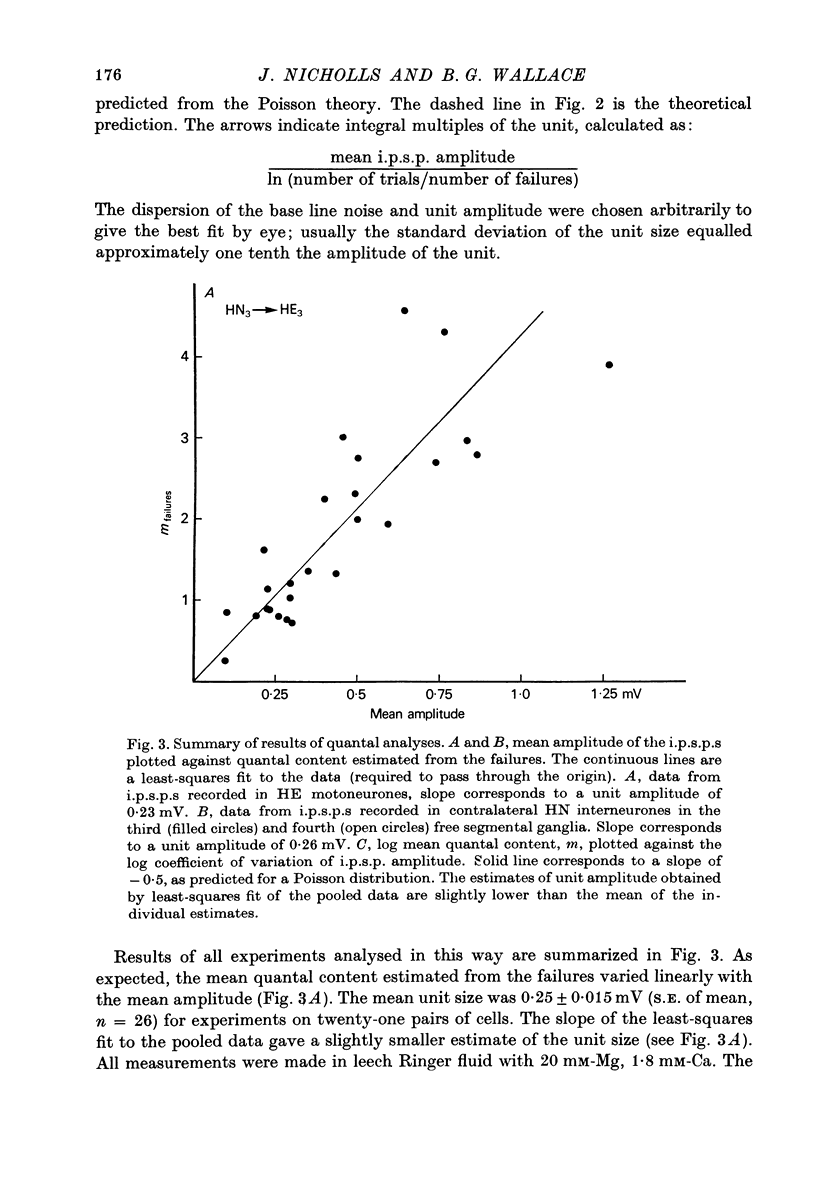
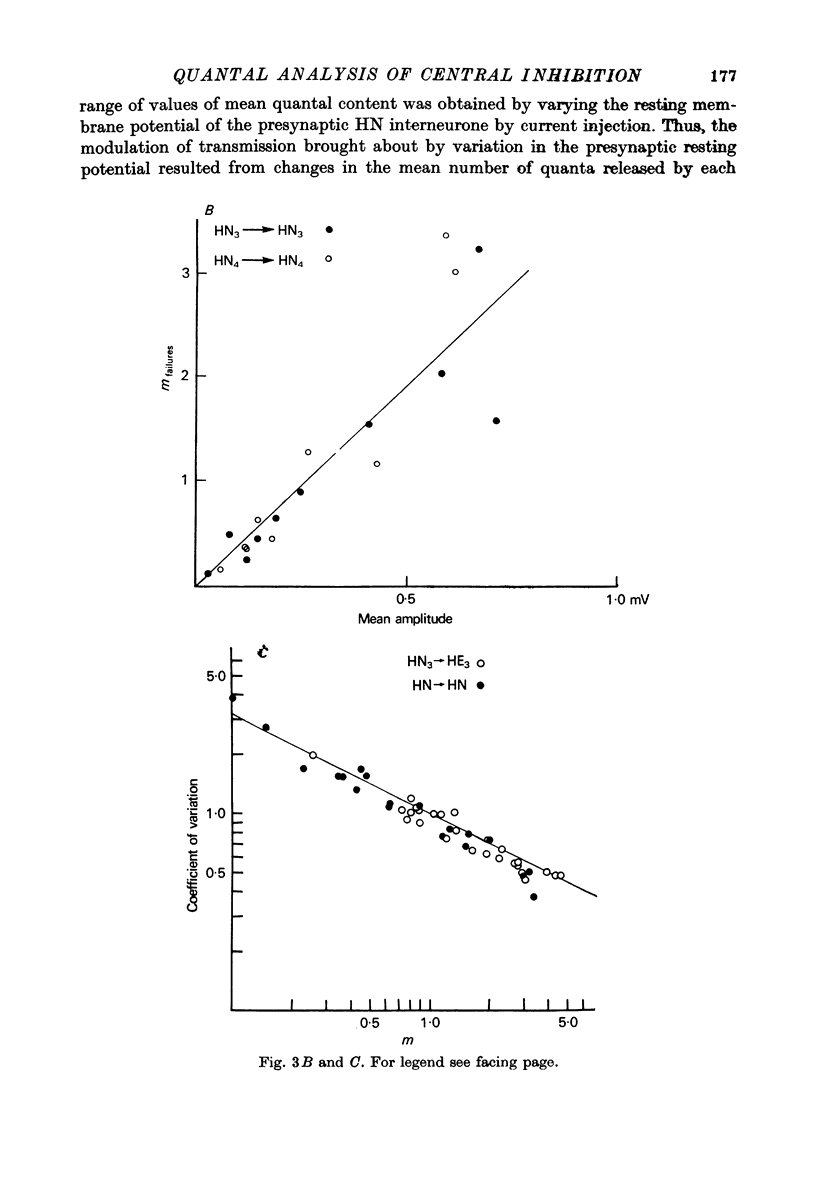
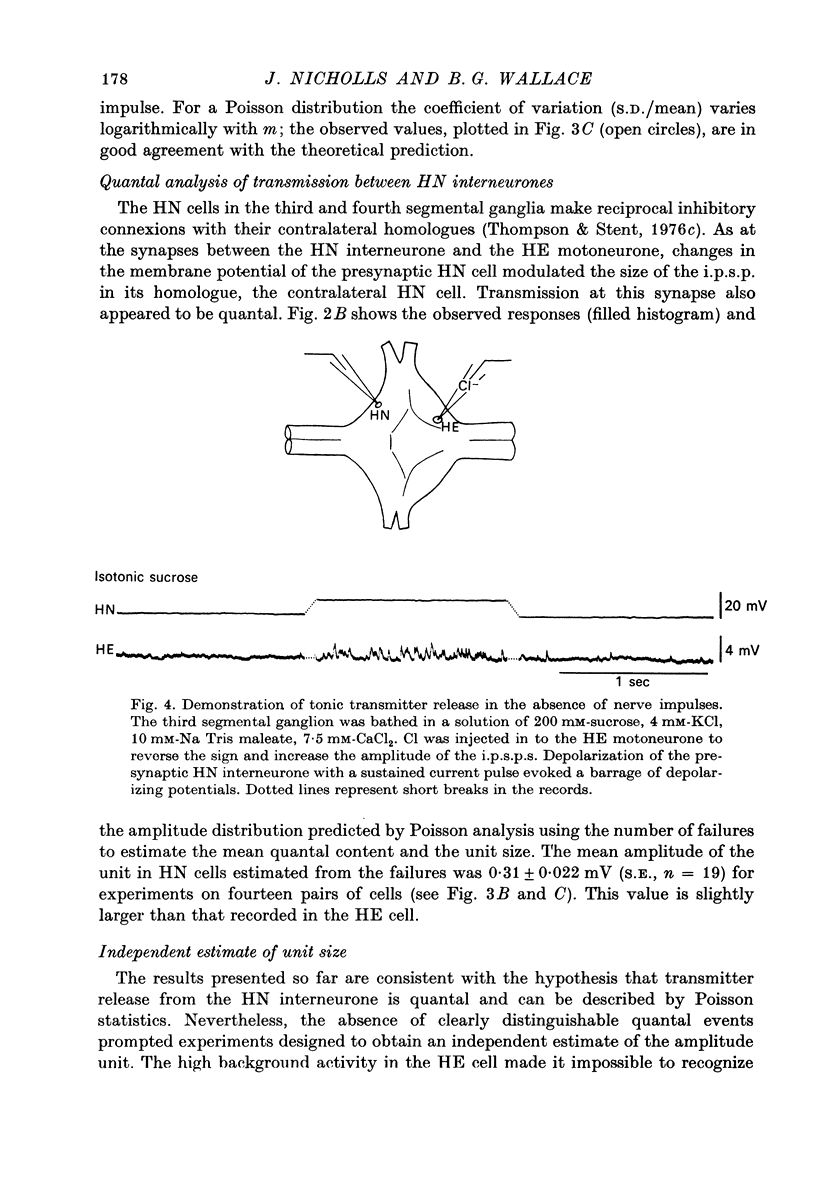
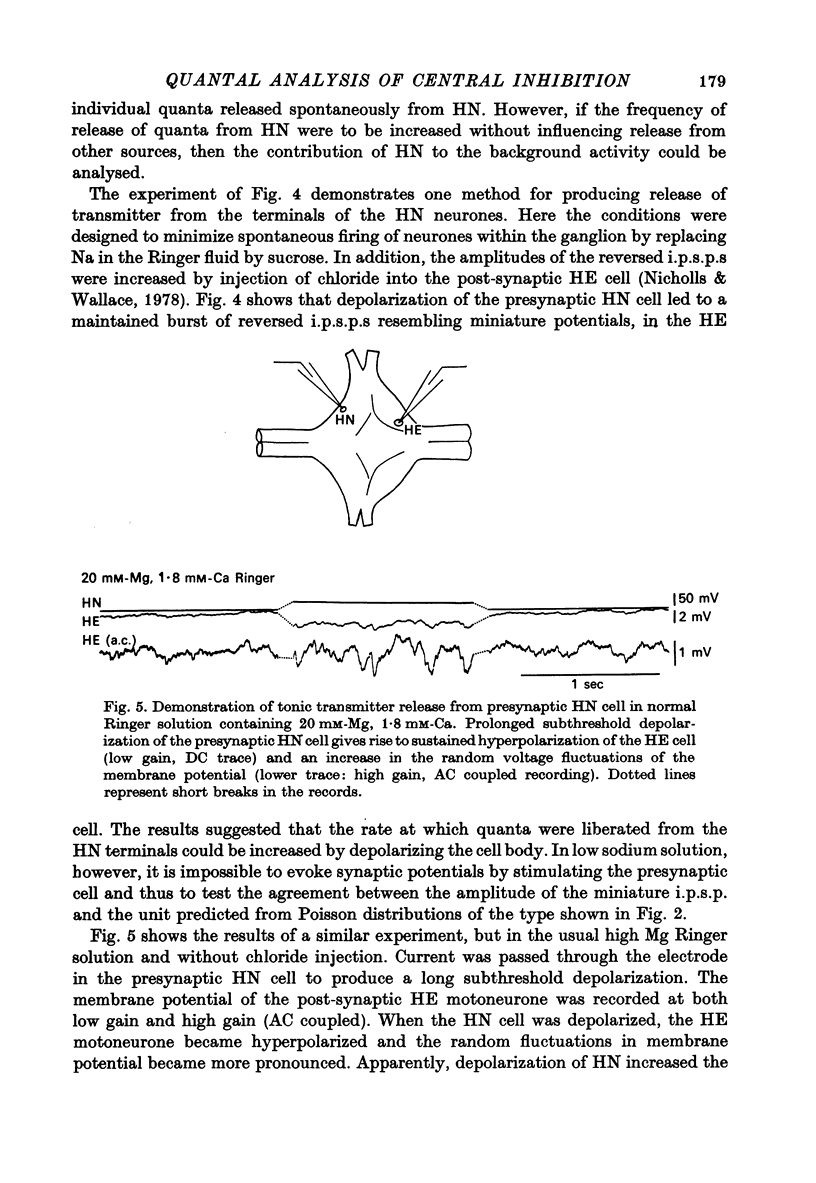
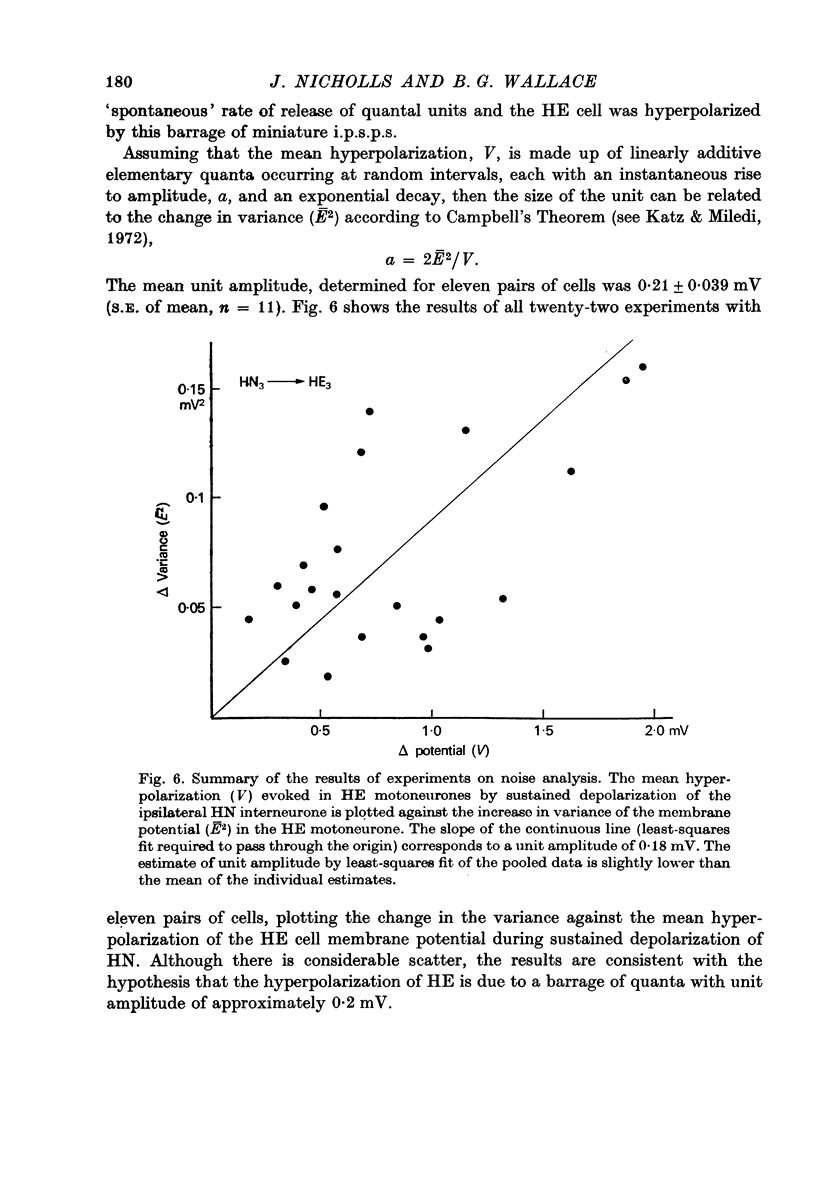
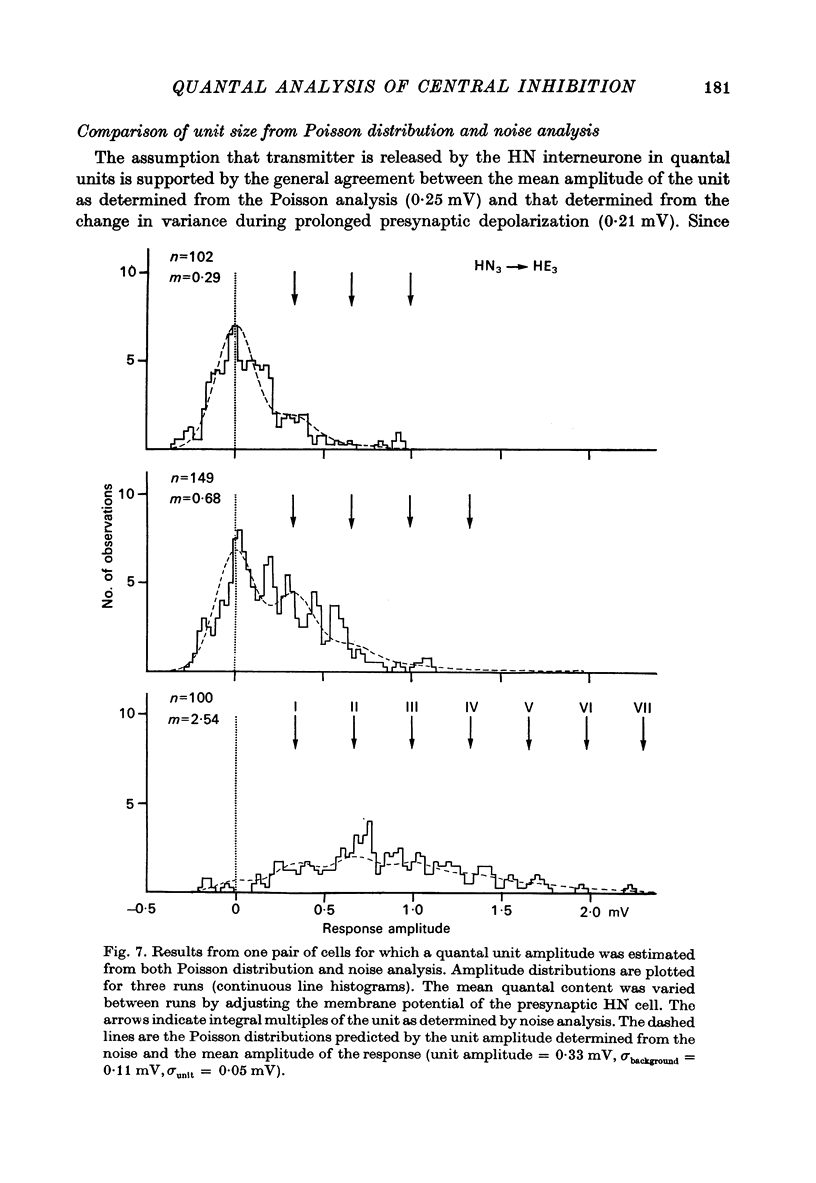
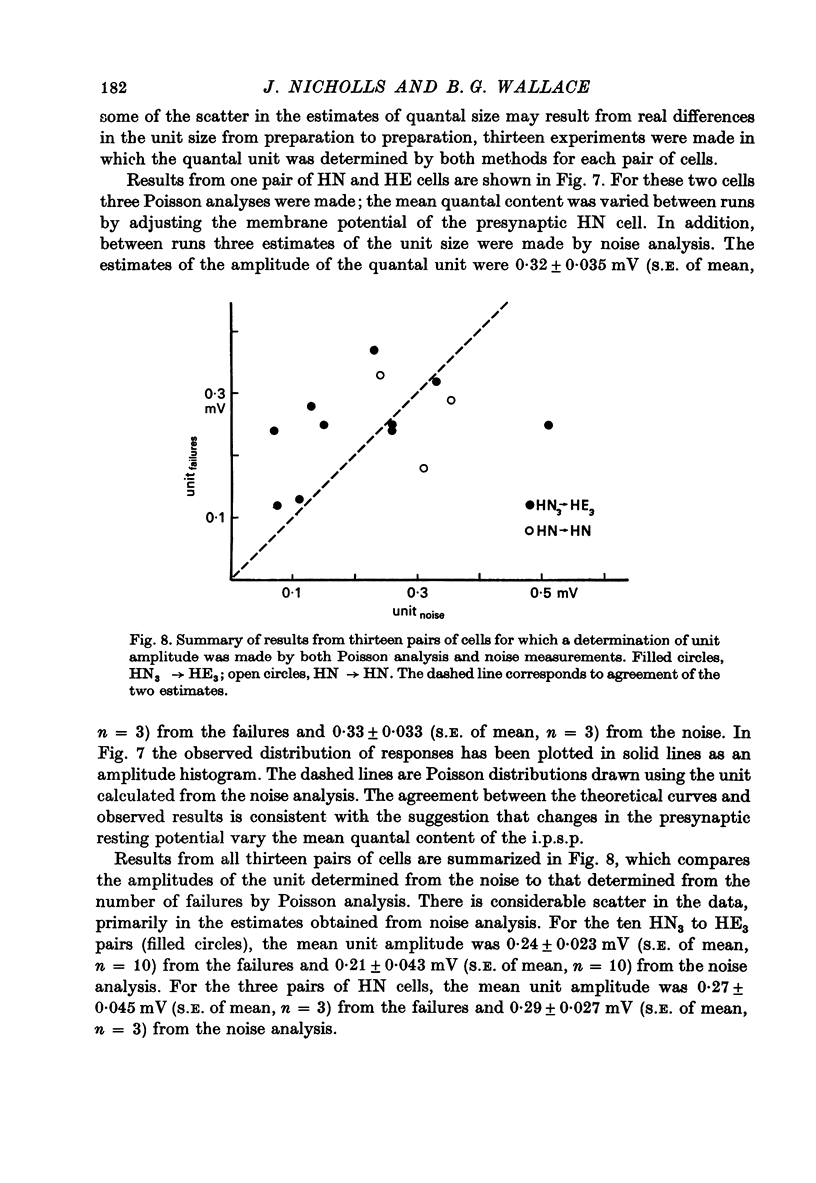
The quantal nature of transmitter release has been analysed at central inhibitory synapses in the leech nervous system between an interneurone (HN) and a motoneurone (HE) that regulate the heartbeat. 1. Ganglia were bathed in leech Ringer fluid containing 20 mM-Mg and 1.8 mM-Ca and the membrane of the presynaptic HN interneurone was hyperpolarized by current injection. Under these conditions successive inhibitory potentials in the HE motoneurone, evoked by impulses in the HN interneurone, showed striking fluctuations in amplitude. 2. Assuming a Poisson distribution of the i.p.s.p.s and estimating the number of failures from the amplitude histograms of the observed responses, the mean size of the quantal unit was estimated as 0.25 +/- 0.015 mV (S.E. of mean, n = 26). When m, the mean number of quanta released per trial, was varied by changing the membrane potential of the presynaptic HN cell (Nicholls & Wallace, 1978), the experimentally observed amplitude distributions could be predicted by the Poisson theory. 3. An independent estimate of the unit size was obtained by noise analysis. A long subthreshold depolarizing pulse applied to the presynaptic HN interneurone evoked a sustained hyperpolarization of the HE motoneurone, apparently caused by an increase in the rate of on-going release of quanta by the HN cell terminals. From the mean change in membrane potential and the increase in variance, the size of the unit was calculated as 0.21 +/- 0.039 mV (S.E. of mean, n = 11). For ten pairs of cells an estimate of unit amplitude was made both from the Poisson analysis and the analysis of variance, again with good agreement. For these cells the estimated unit sizes were 0.24 +/- 0.023 mV (S.E. of mean, n = 10) from the failures and 0.21 +/- 0.043 m V (S.E. of mean, n = 10) from the noise. 4. A similar analysis was made of the inhibitory synaptic potentials evoked in one HN interneurone by stimulation of its contralateral homologue. Transmission again appeared to be qualtal; the mean unit amplitude from Poisson analysis was 0.31 +/- 0.022 mV (S.E. of mean, n = 19) and from the noise 0.29 +/- 0.027 mV (S.E. of mean, n = 3). 5. We conclude that transmitter is released from the terminals of the HN interneurone in quantal units that evoke miniature i.p.s.p.s of about 0.25 mV in the post-synaptic cells. Furthermore, modulation of transmission proudced by variation in the presynaptic resting potential and during presynaptic inhibition results from changes in the mean number of quanta released by each impulse.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. V., Model P. G., Highstein S. M. Stimulation-induced depletion of vesicles, fatigue of transmission and recovery processes at a vertebrate central synapse. Cold Spring Harb Symp Quant Biol. 1976;40:25–35. doi: 10.1101/sqb.1976.040.01.005. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. Non-quantal fluctuations and transmission failures in charge transfer at Ia synapses on spinal motoneurones. J Physiol. 1976 Aug;259(3):689–704. doi: 10.1113/jphysiol.1976.sp011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. Statistical fluctuations in charge transfer at Ia synapses on spinal motoneurones. J Physiol. 1976 Aug;259(3):665–688. doi: 10.1113/jphysiol.1976.sp011488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J. K., Jr, Muller K. J., Nicholls J. G. Persistent modification of synaptic interactions between sensory and motor nerve cells following discrete lesions in the central nervous system of the leech. J Physiol. 1974 Oct;242(2):289–305. doi: 10.1113/jphysiol.1974.sp010708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNO M. QUANTAL COMPONENTS OF EXCITATORY SYNAPTIC POTENTIALS IN SPINAL MOTONEURONES. J Physiol. 1964 Dec;175:81–99. doi: 10.1113/jphysiol.1964.sp007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Brunelli M., Byrne J., Castellucci V. A common presynaptic locus for the synaptic changes underlying short-term habituation and sensitization of the gill-withdrawal reflex in Aplysia. Cold Spring Harb Symp Quant Biol. 1976;40:465–482. doi: 10.1101/sqb.1976.040.01.044. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M. Quantum aspects of central and ganglionic synaptic transmission in vertebrates. Physiol Rev. 1971 Oct;51(4):647–678. doi: 10.1152/physrev.1971.51.4.647. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Nicholls J. G. The properties and connections of nerve cells in leech ganglia maintained in culture. Proc R Soc Lond B Biol Sci. 1976 Oct 29;194(1116):295–311. doi: 10.1098/rspb.1976.0081. [DOI] [PubMed] [Google Scholar]
- Nicholls J., Wallace B. G. Modulation of transmission at an inhibitory synapse in the central nervous system of the leech. J Physiol. 1978 Aug;281:157–170. doi: 10.1113/jphysiol.1978.sp012414. [DOI] [PMC free article] [PubMed] [Google Scholar]


