Abstract
1. Intracellular injections with horseradish peroxidasewere performed in cat alpha-motoneurones supplying various hind-limb muscles. 2. Ten alpha-motoneurones from each of the quadriceps, posterior biceps, gastrocnemius-soleus and anterior tibial pools as well as from the pool supplying the short plantar muscles were collected for morphological analysis of the intramedullary axonal systems including the recurrent axon collaterals. 3. The diameter of the alpha-motor axons showed considerable variation within each motoneurone pool, the total range being from 4.6 to 9.0 micrometer. No significant difference in mean axon diameter was obtained between the different pools. 4. All alpha-motoneurones supplying the short plantar muscles and one single alpha-motoneurone supplying the quadriceps muscle lacked collaterals completely, while the remaining motoneurones gave off one to five collaterals. 5. The number of axon collateral outbulgings, interpreted as synaptic boutons, wihch originated from a single alpha-motoneurone showed large variation within each pool that possessed axon collaterals, the total range being from seventeen of 158. The mean number varied from forty-four (quadriceps) to eighty-two (anterior tibial). 6. The axon collateral outbulgings were distributed not only in the Renshaw cell area ventromedial to the main motor nuclei but also in those parts of the motor nuclei which were located in the vicinity of the parent cell bodies. In the rostrocaudal direction, the outbulgings were distributed within a distances of less than 1 mm around the position of the parent cell bodies. 7. Some physiological implications of the lack of axon collaterals from alpha-motoneurones supplying the short plantar muscles were discussed in relation to the functional characteristics of plantar muscles and motor units.
Full text
PDF

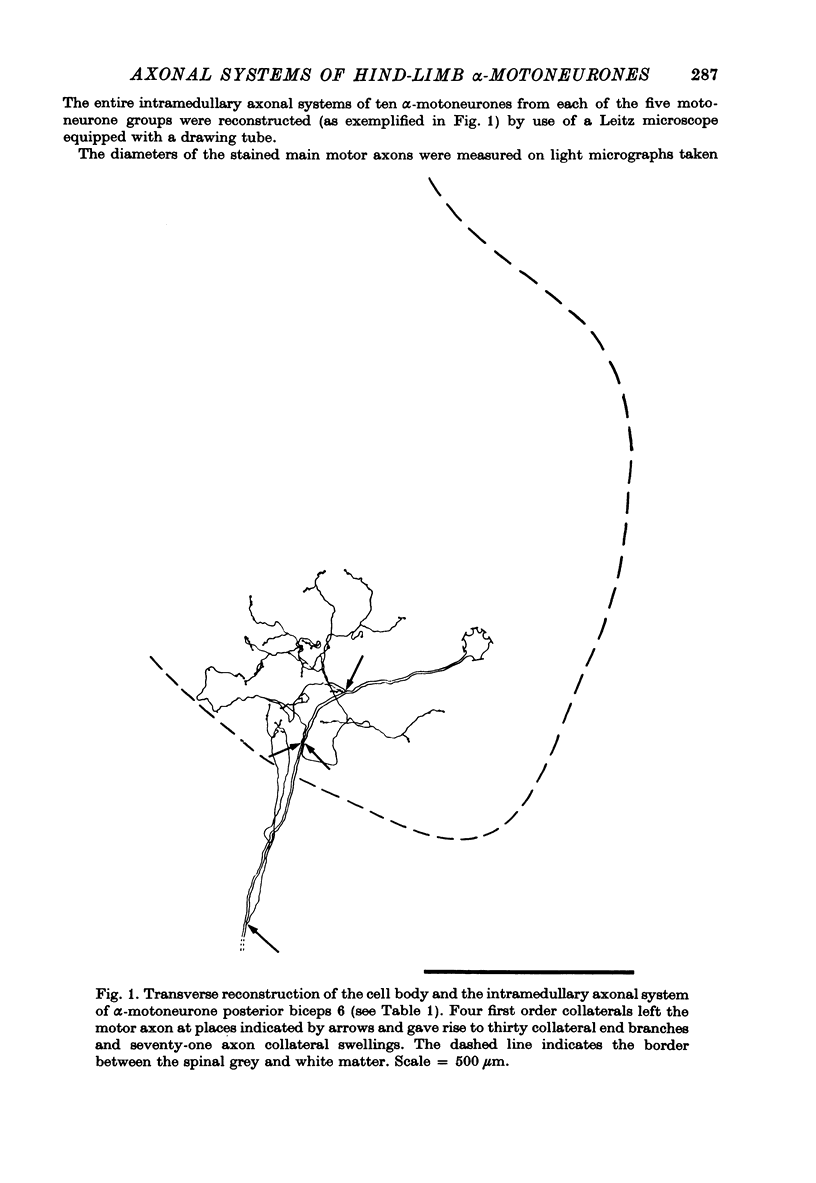

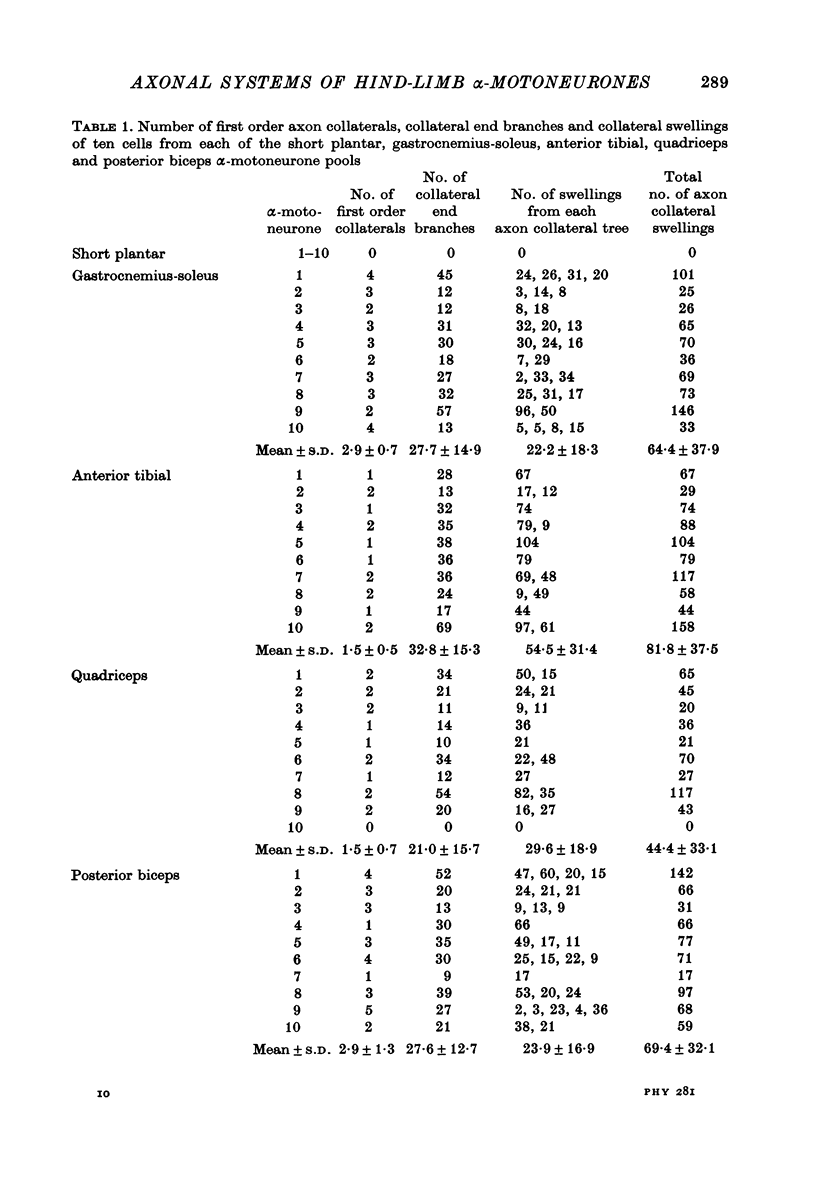




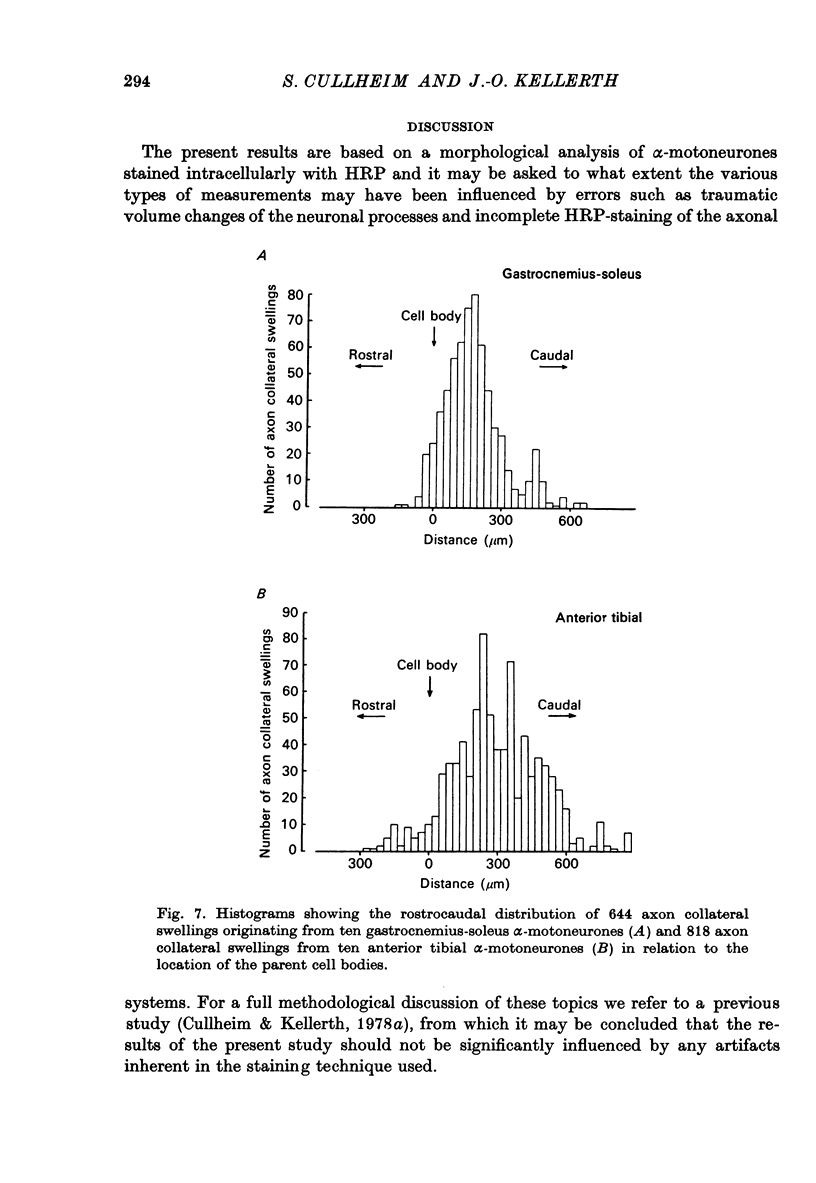





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKS V. B., WILSON V. J. Recurrent inhibition in the cat's spinal cord. J Physiol. 1959 May 19;146(2):380–391. doi: 10.1113/jphysiol.1959.sp006199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Lawrence D. G., Matthews P. B. Antidromic inhibition of presumed fusimotor neurones by repetitive stimulation of the ventral root in the decerebrate cat. Experientia. 1968 Dec 15;24(12):1210–1212. doi: 10.1007/BF02146625. [DOI] [PubMed] [Google Scholar]
- Burke R. E. Motor unit types of cat triceps surae muscle. J Physiol. 1967 Nov;193(1):141–160. doi: 10.1113/jphysiol.1967.sp008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O. A morphological study of the axons and recurrent axon collaterals of cat alpha-motoneurones supplying different functional types of muscle unit. J Physiol. 1978 Aug;281:301–313. doi: 10.1113/jphysiol.1978.sp012423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O. A morphological study of the axons and recurrent axon collaterals of cat sciatic alpha-motoneurons after intracellular staining with horseradish peroxidase. J Comp Neurol. 1978 Apr 1;178(3):537–557. doi: 10.1002/cne.901780309. [DOI] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O., Conradi S. Evidence for direct synaptic interconnections between cat spinal alpha-motoneurons via the recurrent axon collaterals: a morphological study using intracellular injection of horseradish peroxidase. Brain Res. 1977 Aug 19;132(1):1–10. doi: 10.1016/0006-8993(77)90702-8. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., IGGO A., ITO M. Distribution of recurrent inhibition among motoneurones. J Physiol. 1961 Dec;159:479–499. doi: 10.1113/jphysiol.1961.sp006822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol. 1958 Jul 14;142(2):275–291. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958 Dec 4;144(2):271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. Recurrent inhibition of fusimotor neurones exhibiting background discharges in the decerebrate and the spinal cat. J Physiol. 1971 Jul;216(2):419–439. doi: 10.1113/jphysiol.1971.sp009533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., RENKIN B. Net depolarization and discharge rate of motoneurones, as measured by recurrent inhibition. J Physiol. 1961 Oct;158:461–475. doi: 10.1113/jphysiol.1961.sp006780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Holländer H. The section embedding (SE) technique. A new method for the combined light microscopic and electron microscopic examination of central nervous tissue. Brain Res. 1970 May 20;20(1):39–47. doi: 10.1016/0006-8993(70)90152-6. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition from motor axon collatersls in interneurones monosynaptically activated rom la afferents. Brain Res. 1968 Jul;9(2):367–369. doi: 10.1016/0006-8993(68)90240-0. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition of interneurones monosynaptically activated from group Ia afferents. J Physiol. 1971 Jul;215(3):613–636. doi: 10.1113/jphysiol.1971.sp009488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Relative contribution from different nerves to recurrent depression of Ia IPSPs in motoneurones. J Physiol. 1971 Jul;215(3):637–664. doi: 10.1113/jphysiol.1971.sp009489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Rastad J., Westman J. Intracellular application of horseradish peroxidase and its light and electron microscopical appearance in spinocervical tract cells. Brain Res. 1976 Apr 9;105(3):557–562. doi: 10.1016/0006-8993(76)90603-x. [DOI] [PubMed] [Google Scholar]
- KUNO M. Excitability following antidromic activation in spinal motoneurones supplying red muscles. J Physiol. 1959 Dec;149:374–393. doi: 10.1113/jphysiol.1959.sp006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D., Sjöholm H. Recruitment and firing rate modulation of motor unit tension in a small muscle of the cat's foot. Brain Res. 1975 Nov 7;98(1):57–72. doi: 10.1016/0006-8993(75)90509-0. [DOI] [PubMed] [Google Scholar]
- Lindström S., Schomburg E. D. Recurrent inhibition from motor axon collaterals of ventral spinocerebellar tract neurones. Acta Physiol Scand. 1973 Aug;88(4):505–515. doi: 10.1111/j.1748-1716.1973.tb05479.x. [DOI] [PubMed] [Google Scholar]
- Pugh P. M., Stone S. L. The effect of 2,4-dinitrophenol and related compounds on bile secretion. J Physiol. 1968 Sep;198(1):39–49. doi: 10.1113/jphysiol.1968.sp008592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMANES G. J. The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol. 1951 Apr;94(2):313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- Ryall R. W., Piercey M. F., Polosa C. Intersegmental and intrasegmental distribution of mutual inhibition of Renshaw cells. J Neurophysiol. 1971 Jul;34(4):700–707. doi: 10.1152/jn.1971.34.4.700. [DOI] [PubMed] [Google Scholar]
- Ryall R. W. Renshaw cell mediated inhibition of Renshaw cells: patterns of excitation and inhibition from impulses in motor axon collaterals. J Neurophysiol. 1970 Mar;33(2):257–270. doi: 10.1152/jn.1970.33.2.257. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Inhibition and the Renshaw cell. A structural critique. Brain Behav Evol. 1971;4(1):53–93. doi: 10.1159/000125424. [DOI] [PubMed] [Google Scholar]
- Snow P. J., Rose P. K., Brown A. G. Tracing axons and axon collaterals of spinal neurons using intracellular injection of horseradish peroxidase. Science. 1976 Jan 23;191(4224):312–313. doi: 10.1126/science.54936. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., TALBOT W. H., DIECKE F. P. Distribution of recurrent facilitation and inhibition in cat spinal cord. J Neurophysiol. 1960 Mar;23:144–153. doi: 10.1152/jn.1960.23.2.144. [DOI] [PubMed] [Google Scholar]


