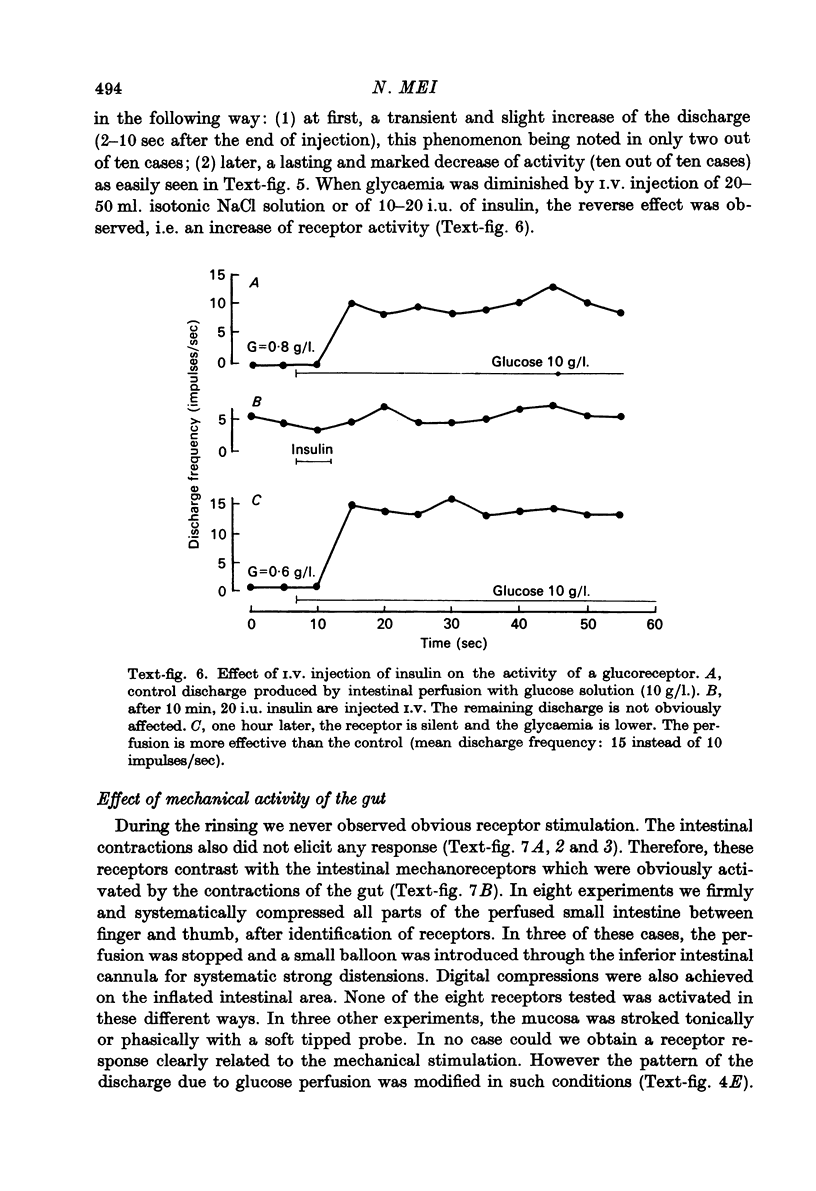
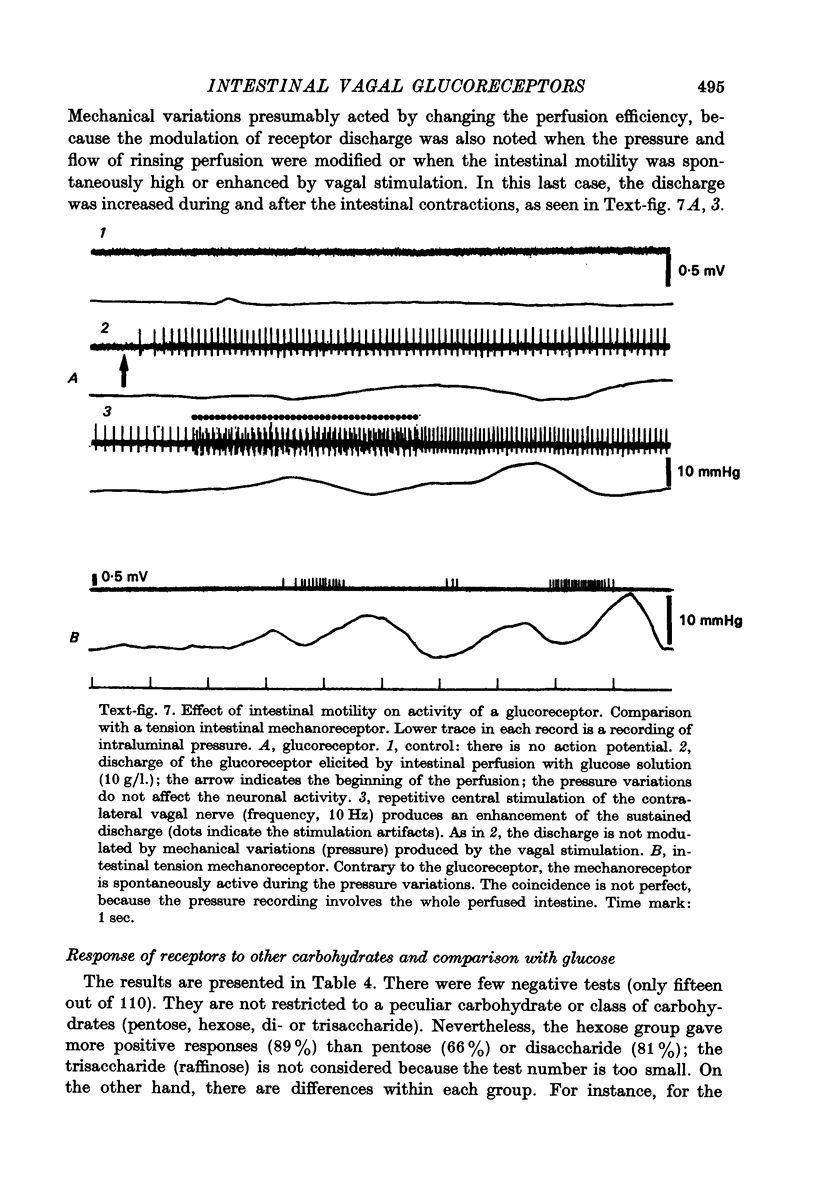
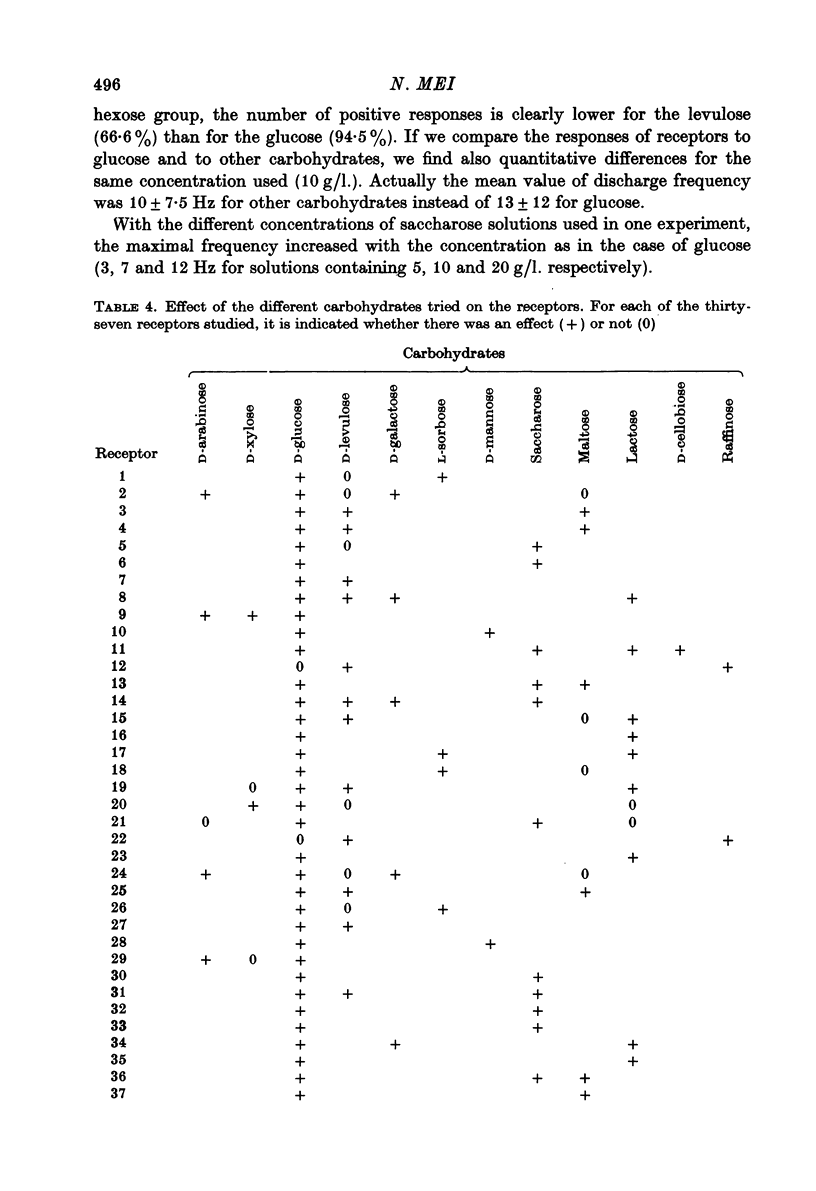
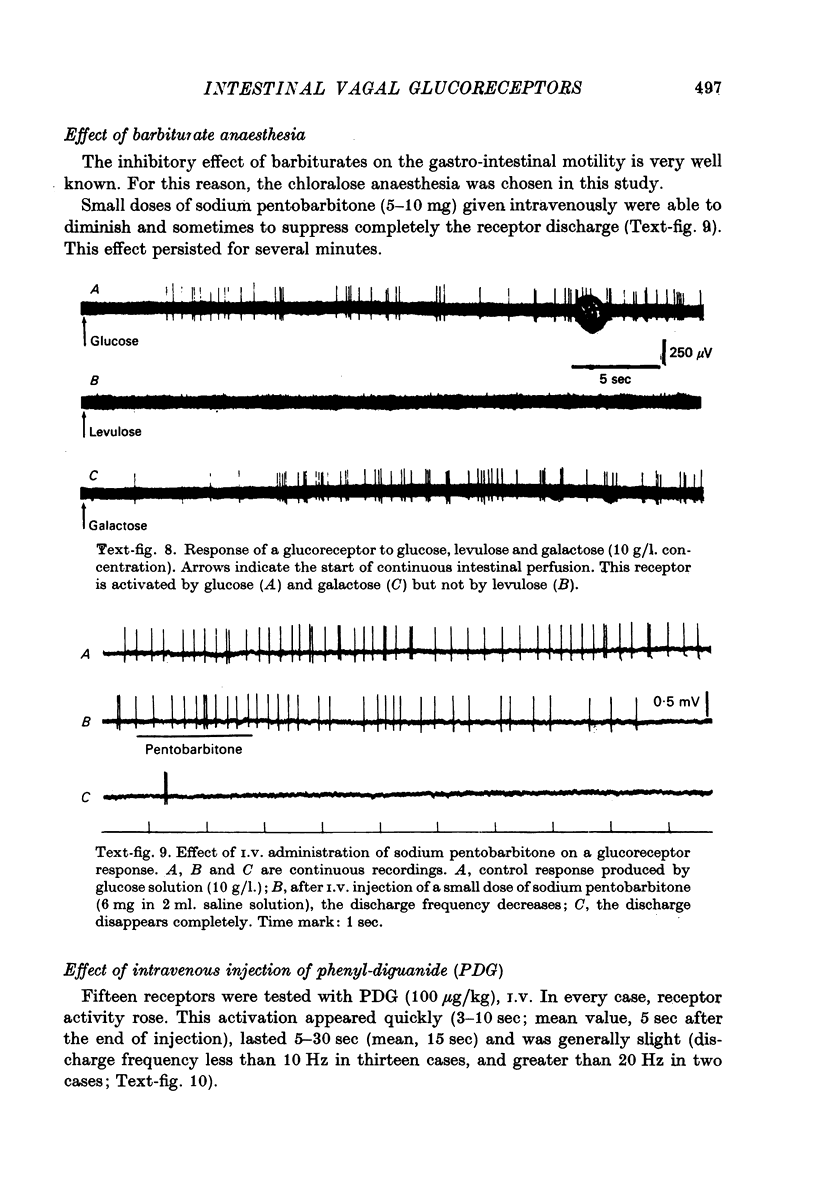
Abstract
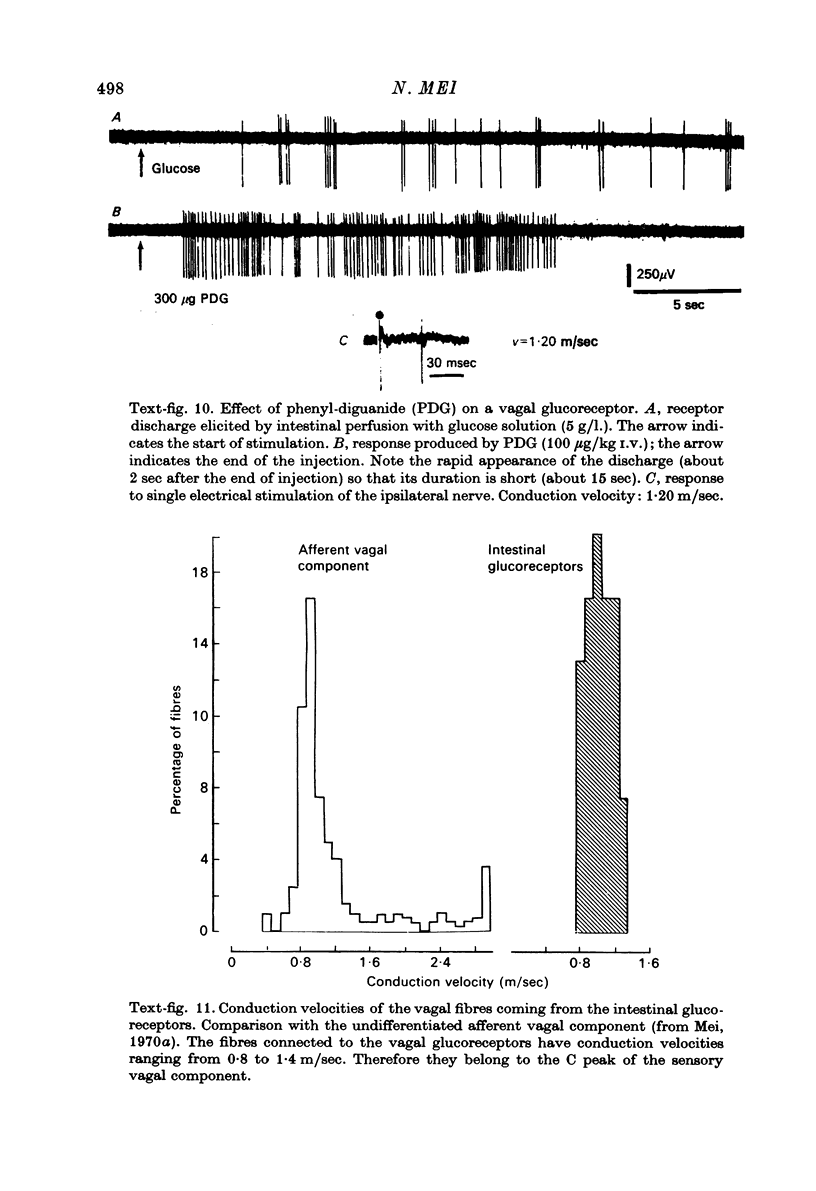
1. In anaesthetized cats, the unitary activity of seventy-eight sensory vagal neurones was recorded in nodose ganglia by means of extracellular glass microelectrodes. 2. These neurones were stimulated by perfusion of the small intestine (duodenum and first part of jejunum) with glucose or other different carbohydrates at concentrations of 1--20 g/l. (i.e. 55--1100 m-osmole/l.). 3. The neurones were slowly adapting to stimulation and their discharge frequency was always low (1--30 Hz). 4. The activity of these neurones depended on the particular carbohydrate used and on its concentration: the discharge frequency generally increased when the concentration rose. 5. The neurones were of the C type (conduction velocities: 0.8--1.4 m/sec; mean, 1.1 m/sec). 6. In contrast with the known neurones connected to the gastro-intestinal tension receptors, they were not obviously activated by intestinal contractions or distensions. 7. In the same way, the stimuli which produced the response of other known endings, i.e. the mucosal receptors, were not effective; these stimuli included in particular stroking of the mucosa, over-distension of the bowel, intestinal perfusion with alkaline or acid solutions. On the other hand, the use of substances other than glucose (KCl and NaCl of the same osmolarity) showed that the osmotic pressure was not directly related to the receptor activation. 8. Therefore it is proposed to call the endings corresponding to these neurones 'glucoreceptors'. 9. The effect of glycaemia and intestinal motility were also studied. These variables acted presumably by changing the intestinal absorption rate. 10. The functional characteristics of the glucoreceptors (in particular the short latency of their response) strongly suggested that they were located close to the intestinal epithelium. 11. An ultrastructural study was performed in an attempt to identify the histological site of the receptors. Many non-medullated fibres were observed in the villi, especially beneath the epithelial layer. They gave complex branchings with abundant swellings. Some of them, at least, belonged to the vagal sensory component, because they were less numerous after unilateral selective sensory vagotomy. Therefore these complex endings could serve as the vagal glucoreceptors. 12. The roles of vagal intestinal glucoreceptors are discussed. Their functional characteristics as well as the clinical and experimental data suggest that they may be involved in the regulation of different types of alimentary behaviour (hunger, thirst, alliesthesia) and energy balance.
Full text
PDF


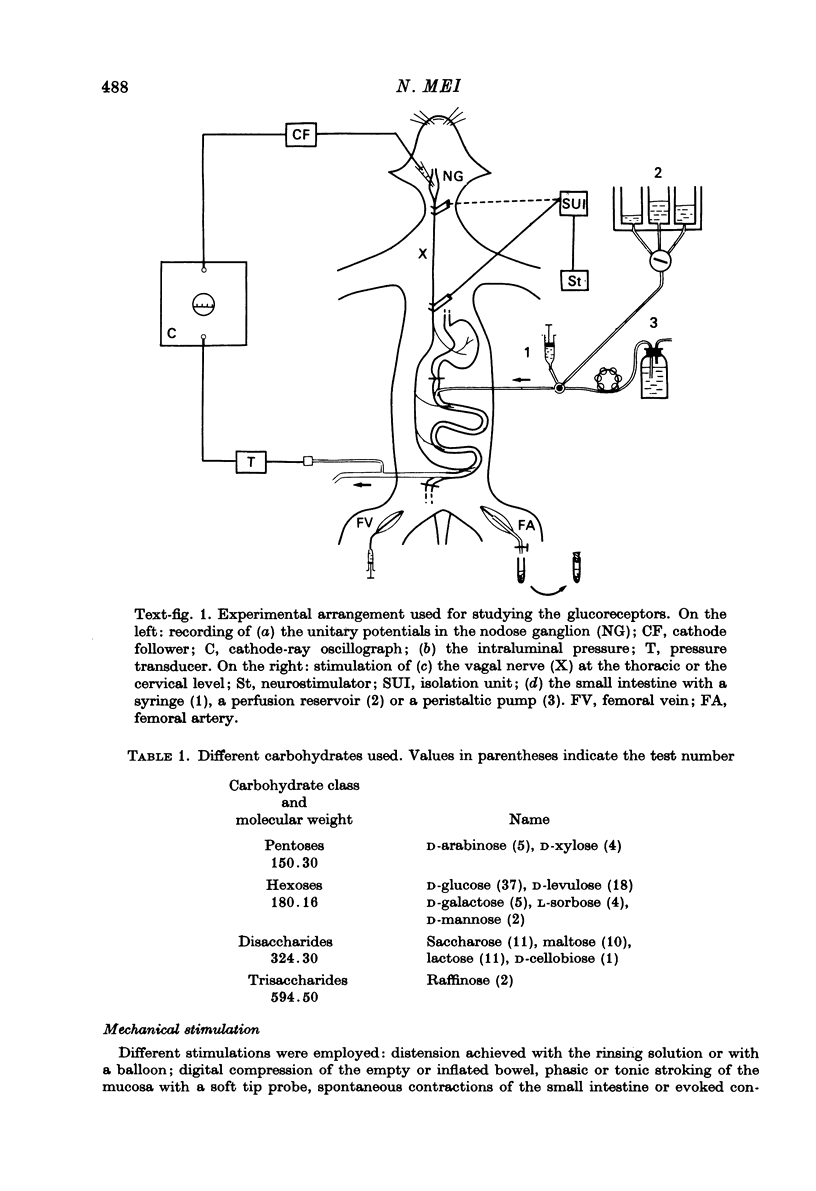

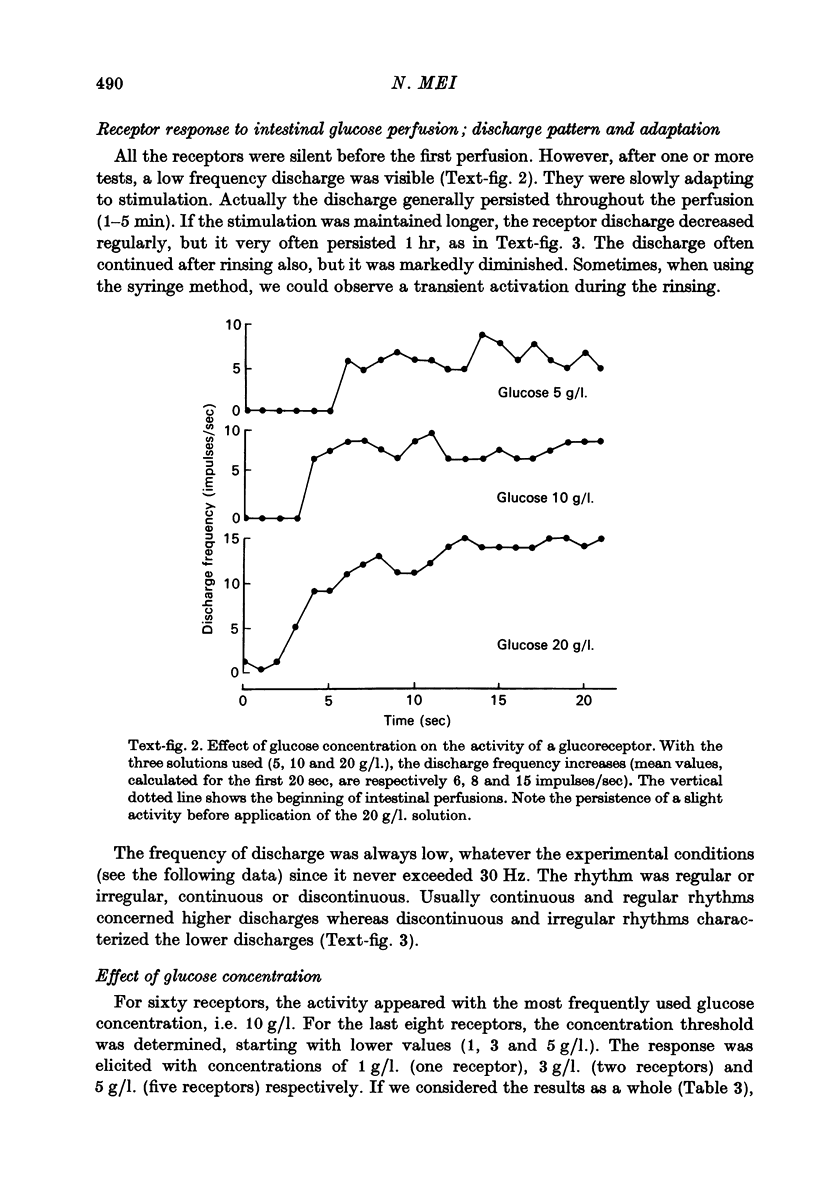
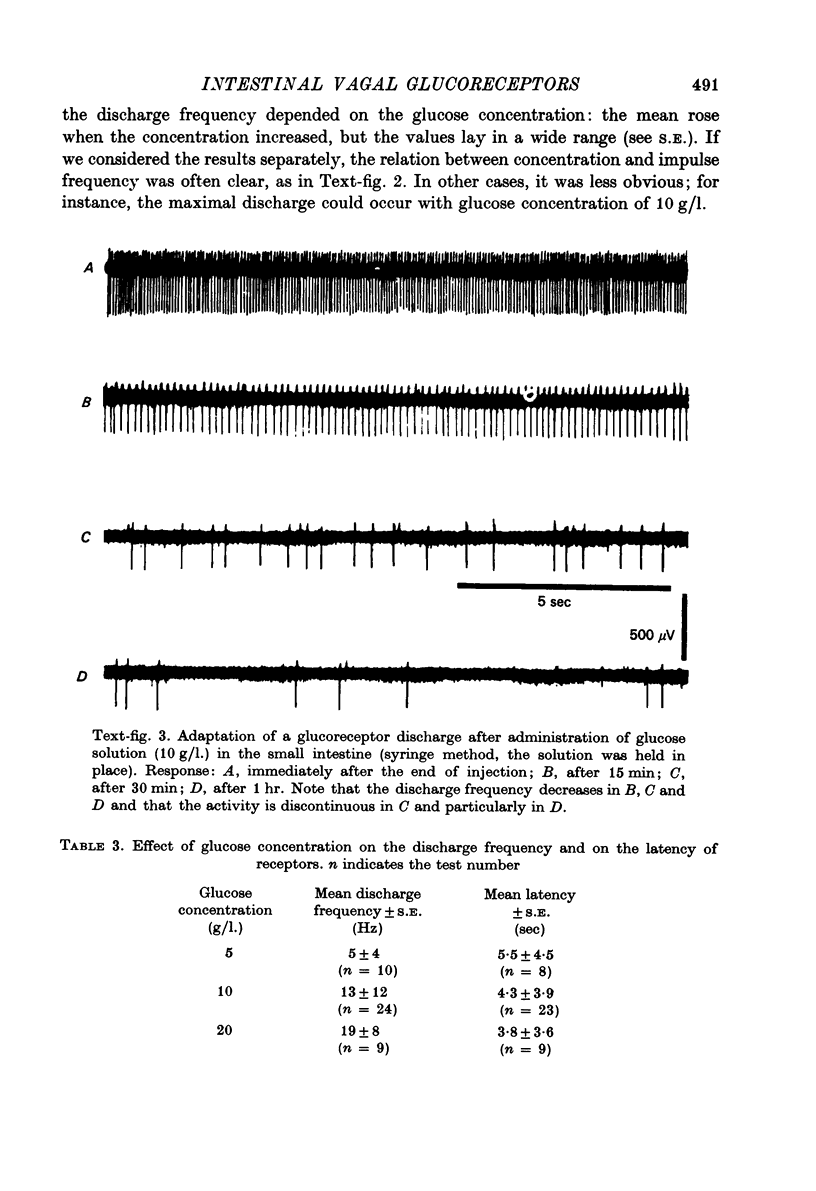
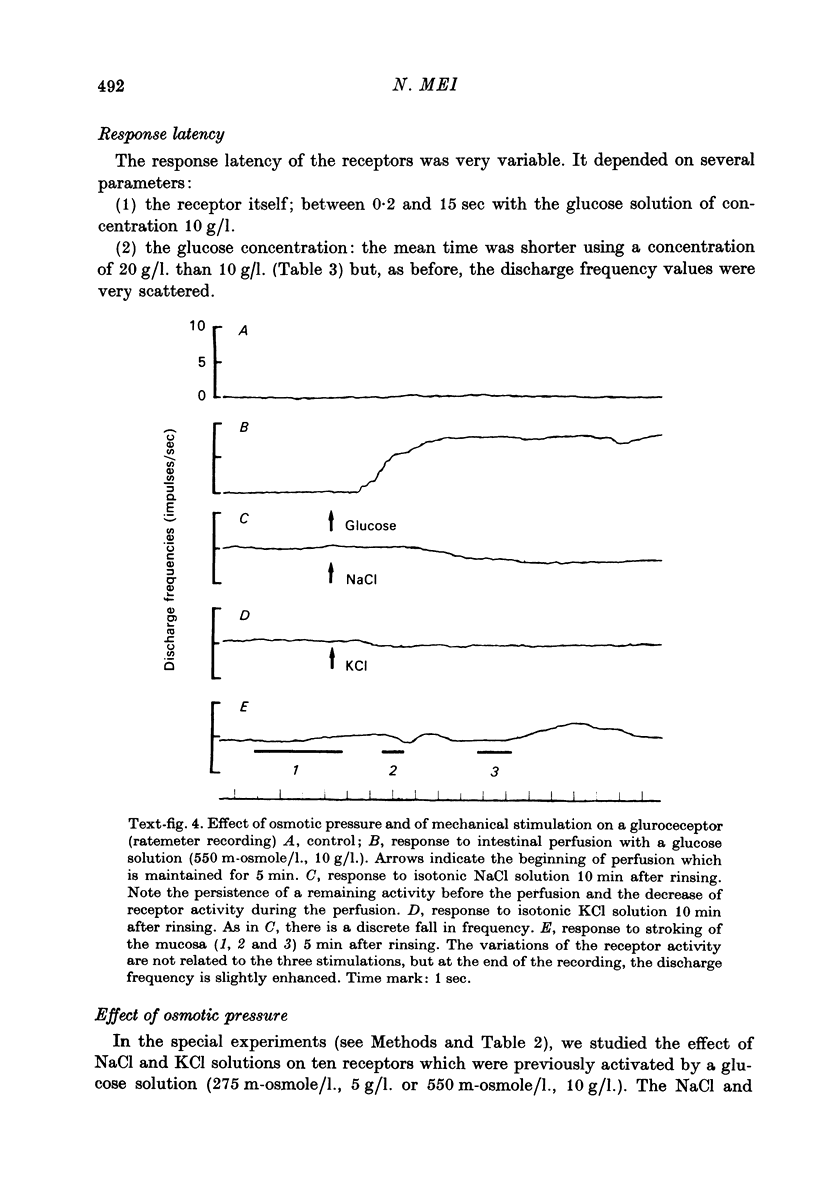








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGOSTONI E., CHINNOCK J. E., DE DALY M. B., MURRAY J. G. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol. 1957 Jan 23;135(1):182–205. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi A., Niijima A., Jacobs H. L. An hepatic osmoreceptor mechanism in the rat: electrophysiological and behavioral studies. Am J Physiol. 1976 Oct;231(4):1043–1049. doi: 10.1152/ajplegacy.1976.231.4.1043. [DOI] [PubMed] [Google Scholar]
- Andrews C. J., Andrews W. H. Receptors, activated by acid, in the duodenal wall of rabbits. Q J Exp Physiol Cogn Med Sci. 1971 Oct;56(4):221–230. doi: 10.1113/expphysiol.1971.sp002123. [DOI] [PubMed] [Google Scholar]
- Anner B., Ferrero J., Jirounek P., Jones G. J., Salamin A., Straub R. W. Sodium-dependent influx of orthophosphate in mammalian non-myelinated nerve. J Physiol. 1976 Sep;260(3):667–686. doi: 10.1113/jphysiol.1976.sp011538. [DOI] [PMC free article] [PubMed] [Google Scholar]
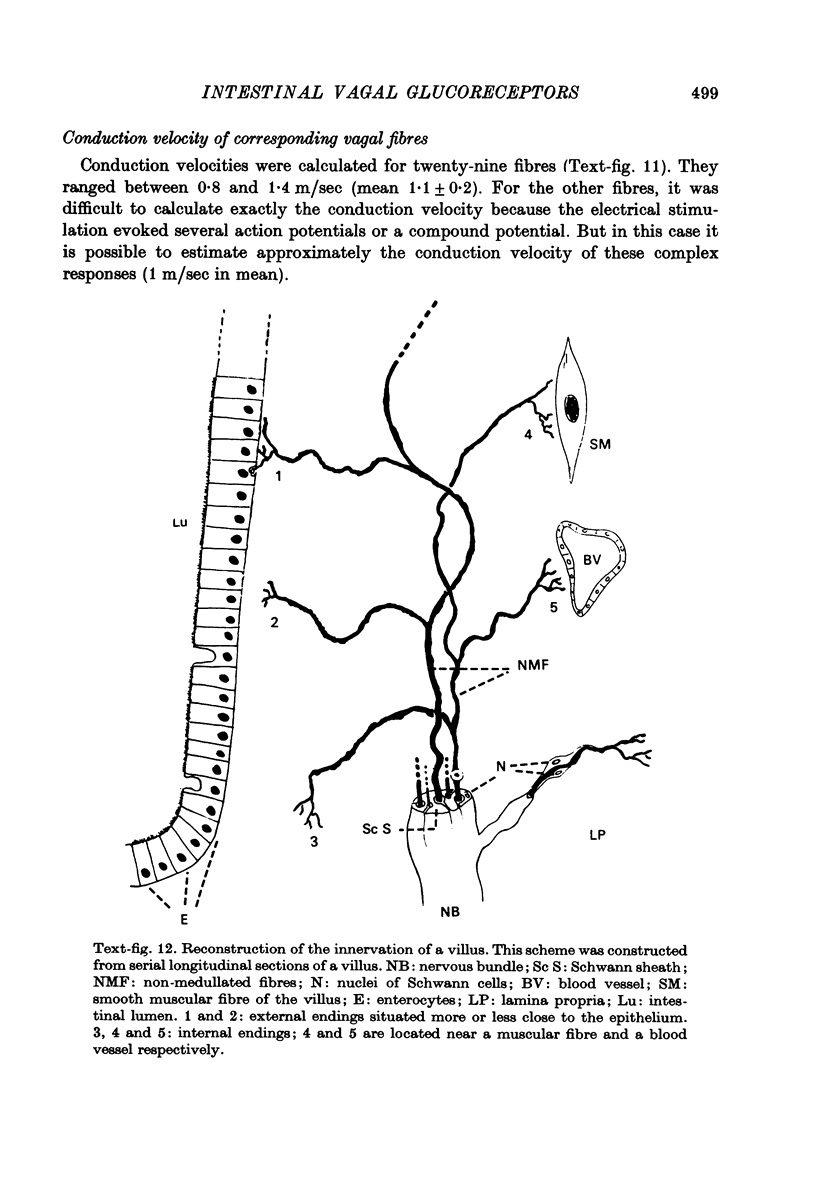
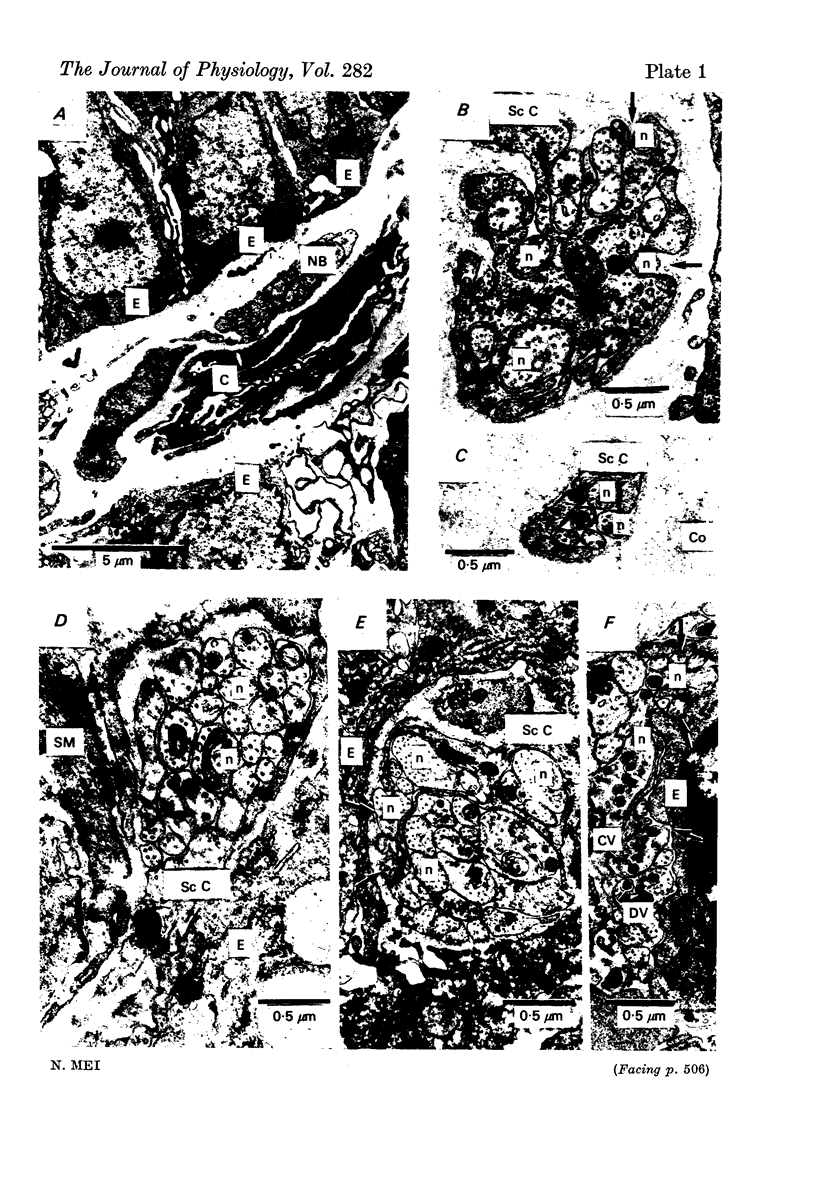
- Anner B., Ferrero J., Jirounek P., Straub R. W. Uptake of orthophosphate by rabbit vagus nerve fibres. J Physiol. 1975 Jun;247(3):759–771. doi: 10.1113/jphysiol.1975.sp010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Phosphorus metabolism of intact crab nerve and its relation to the active transport of ions. J Physiol. 1965 Sep;180(2):383–423. doi: 10.1113/jphysiol.1965.sp007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann K., de Rouffignac C., Roinel N., Rumrich G., Ullrich K. J. Renal phosphate transport: inhomogeneity of local proximal transport rates and sodium dependence. Pflugers Arch. 1975;356(4):287–298. doi: 10.1007/BF00580003. [DOI] [PubMed] [Google Scholar]
- Bell F. R., Watson D. J. The influence of gastric distension and the duodenal infusate on the pattern of stomach (abomasal) emptying in the preruminant calf. J Physiol. 1976 Jul;259(2):445–456. doi: 10.1113/jphysiol.1976.sp011475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner W., Kinne R., Murer H. Phosphate transport into brush-border membrane vesicles isolated from rat small intestine. Biochem J. 1976 Dec 15;160(3):467–474. doi: 10.1042/bj1600467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Lamb J. F. Proceedings: Na-dependent phosphate transport in cultured cells. J Physiol. 1975 Sep;251(1):58P–59P. [PubMed] [Google Scholar]
- Cabanac M., Duclaux R. Alliesthésie olfacto-gustative et prise alimentaire chez l'homme. J Physiol (Paris) 1973;66(2):113–135. [PubMed] [Google Scholar]
- Clarke G. D., Davison J. S. Proceedings: Vagal afferent nerve endings in the gastric antral mucosa of the rat. J Physiol. 1974 May;239(1):41P–42P. [PubMed] [Google Scholar]
- Coty W. A., Pedersen P. L. Phosphate transport in rat liver mitochondria. Kinetics and energy requirements. J Biol Chem. 1974 Apr 25;249(8):2593–2598. [PubMed] [Google Scholar]
- Davison J. S. Response of single vagal afferent fibres to mechanical and chemical stimulation of the gastric and duodenal mucosa in cats. Q J Exp Physiol Cogn Med Sci. 1972 Oct;57(4):405–416. doi: 10.1113/expphysiol.1972.sp002176. [DOI] [PubMed] [Google Scholar]
- Duclaux R., Mei N., Ranieri F. Conduction velocity along the afferent vagal dendrites: a new type of fibre. J Physiol. 1976 Sep;260(2):487–495. doi: 10.1113/jphysiol.1976.sp011527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero J., Jirounek P., Rouiller M., Salamin A., Straub R. W. Efflux of inorganic phosphate from rabbit vagus in Locke and Na-free Locke [proceedings]. J Physiol. 1976 Dec;263(1):215P–216P. [PubMed] [Google Scholar]
- Floyd K., Hick V. E., Morrison J. F. Mechanosensitive afferent units in the hypogastric nerve of the cat. J Physiol. 1976 Jul;259(2):457–471. doi: 10.1113/jphysiol.1976.sp011476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd K., Morrison J. F. Splanchnic mechanoreceptors in the dog. Q J Exp Physiol Cogn Med Sci. 1974 Oct;59(4):361–366. doi: 10.1113/expphysiol.1974.sp002279. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., Ezdinli E. Z., Javid R. Effect of vagotomy and vagal stimulation on insulin secretion. Diabetes. 1967 Jul;16(7):443–448. doi: 10.2337/diab.16.7.443. [DOI] [PubMed] [Google Scholar]
- HARRISON H. E., HARRISON H. C. Sodium, potassium, and intestinal transport of glucose, 1-tyrosine, phosphate, and calcium. Am J Physiol. 1963 Jul;205:107–111. doi: 10.1152/ajplegacy.1963.205.1.107. [DOI] [PubMed] [Google Scholar]
- Harding R., Leek B. F. Gastro-duodenal receptor responses to chemical and mechanical stimuli, investigated by a 'single fibre' technique. J Physiol. 1972 Apr;222(2):139P–140P. [PubMed] [Google Scholar]
- Henderson J. R., Jefferys D. B., Jones R. H., Stanley D. The effect of atropine on the insulin release caused by oral and intravenous glucose in human subjects. Acta Endocrinol (Copenh) 1976 Dec;83(4):772–780. doi: 10.1530/acta.0.0830772. [DOI] [PubMed] [Google Scholar]
- Hoffmann N., Thees M., Kinne R. Phosphate transport by isolated renal brush border vesicles. Pflugers Arch. 1976 Mar 30;362(2):147–156. doi: 10.1007/BF00583641. [DOI] [PubMed] [Google Scholar]
- IGGO A. Gastric mucosal chemoreceptors with vagal afferent fibres in the cat. Q J Exp Physiol Cogn Med Sci. 1957 Oct;42(4):398–409. doi: 10.1113/expphysiol.1957.sp001284. [DOI] [PubMed] [Google Scholar]
- IGGO A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955 Jun 28;128(3):593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek B. F. Abdominal and pelvic visceral receptors. Br Med Bull. 1977 May;33(2):163–168. doi: 10.1093/oxfordjournals.bmb.a071417. [DOI] [PubMed] [Google Scholar]
- Medveczky N., Rosenberg H. Phosphate transport in Escherichia coli. Biochim Biophys Acta. 1971 Aug 13;241(2):494–506. doi: 10.1016/0005-2736(71)90048-4. [DOI] [PubMed] [Google Scholar]
- Mei N., Boyer A., Condamin M. Etude comparée des deux prolongements de la cellule sensitive vagale. C R Seances Soc Biol Fil. 1971;165(12):2371–2374. [PubMed] [Google Scholar]
- Mei N. Disposition anatomique et propriétés électrophysiologiques des neurones sensitifs vagaux chez le chat. Exp Brain Res. 1970;11(5):465–479. [PubMed] [Google Scholar]
- Mei N. Mécanorécepteurs vagaux cardio-vasculaires et respiratoires chez le chat. Exp Brain Res. 1970;11(5):480–501. [PubMed] [Google Scholar]
- Mei N. Mécanorécepteurs vagaux digestifs chez le chat. Exp Brain Res. 1970;11(5):502–514. [PubMed] [Google Scholar]
- Morrison J. F. Splanchnic slowly adapting mechanoreceptors with punctate receptive fields in the mesentery and gastrointestinal tract of the cat. J Physiol. 1973 Sep;233(2):349–361. doi: 10.1113/jphysiol.1973.sp010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niijima A. Afferent discharges from osmoreceptors in the liver of the guinea pig. Science. 1969 Dec 19;166(3912):1519–1520. doi: 10.1126/science.166.3912.1519. [DOI] [PubMed] [Google Scholar]
- Niijima A. Afferent impulse discharges from glucoreceptors in the liver of the guinea pig. Ann N Y Acad Sci. 1969 May 15;157(2):690–700. doi: 10.1111/j.1749-6632.1969.tb12914.x. [DOI] [PubMed] [Google Scholar]
- PAINTAL A. S. A study of gastric stretch receptors; their role in the peripheral mechanism of satiation of hunger and thirst. J Physiol. 1954 Nov 29;126(2):255–270. doi: 10.1113/jphysiol.1954.sp005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. Responses from mucosal mechanoreceptors in the small intestine of the cat. J Physiol. 1957 Dec 31;139(3):353–368. doi: 10.1113/jphysiol.1957.sp005896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintal A. S. Vagal sensory receptors and their reflex effects. Physiol Rev. 1973 Jan;53(1):159–227. doi: 10.1152/physrev.1973.53.1.159. [DOI] [PubMed] [Google Scholar]
- Ranieri F., Crousillat J., Mei N. Etude électrophysiologique et histologique des fibres afférentes splanchniques. Arch Ital Biol. 1975 Dec;113(4):354–373. [PubMed] [Google Scholar]
- Ranieri F., Mei N., Crousillat J. Les afférences splanchniques provenant des mécanorécepteurs gastro-intestinaux et péritonéaux. Exp Brain Res. 1973 Jan 29;16(3):276–290. doi: 10.1007/BF00233331. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Straub R. W. Increase in efflux of inorganic phosphate during electrical activity in small non-myelinated nerve fibres. J Physiol. 1978 Jan;274:539–548. doi: 10.1113/jphysiol.1978.sp012165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozé C., Couturier D., Chariot J., Debray C. Inhibition of gastric electrical and mechanical activity by intraduodenal agents in pigs and the effects of vagotomy. Digestion. 1977;15(6):526–539. doi: 10.1159/000198043. [DOI] [PubMed] [Google Scholar]
- SHARMA K. N., NASSET E. S. Electrical activity in mesenteric nerves after perfusion of gut lumen. Am J Physiol. 1962 Apr;202:725–730. doi: 10.1152/ajplegacy.1962.202.4.725. [DOI] [PubMed] [Google Scholar]
- Straub R. W., Ferrero J., Jirounek P., Rouiller M., Salamin A. Sodium-dependent transport of orthophosphate in nerve fibres. Adv Exp Med Biol. 1977;81:333–344. doi: 10.1007/978-1-4613-4217-5_33. [DOI] [PubMed] [Google Scholar]



