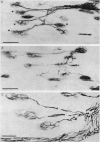
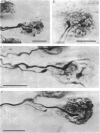
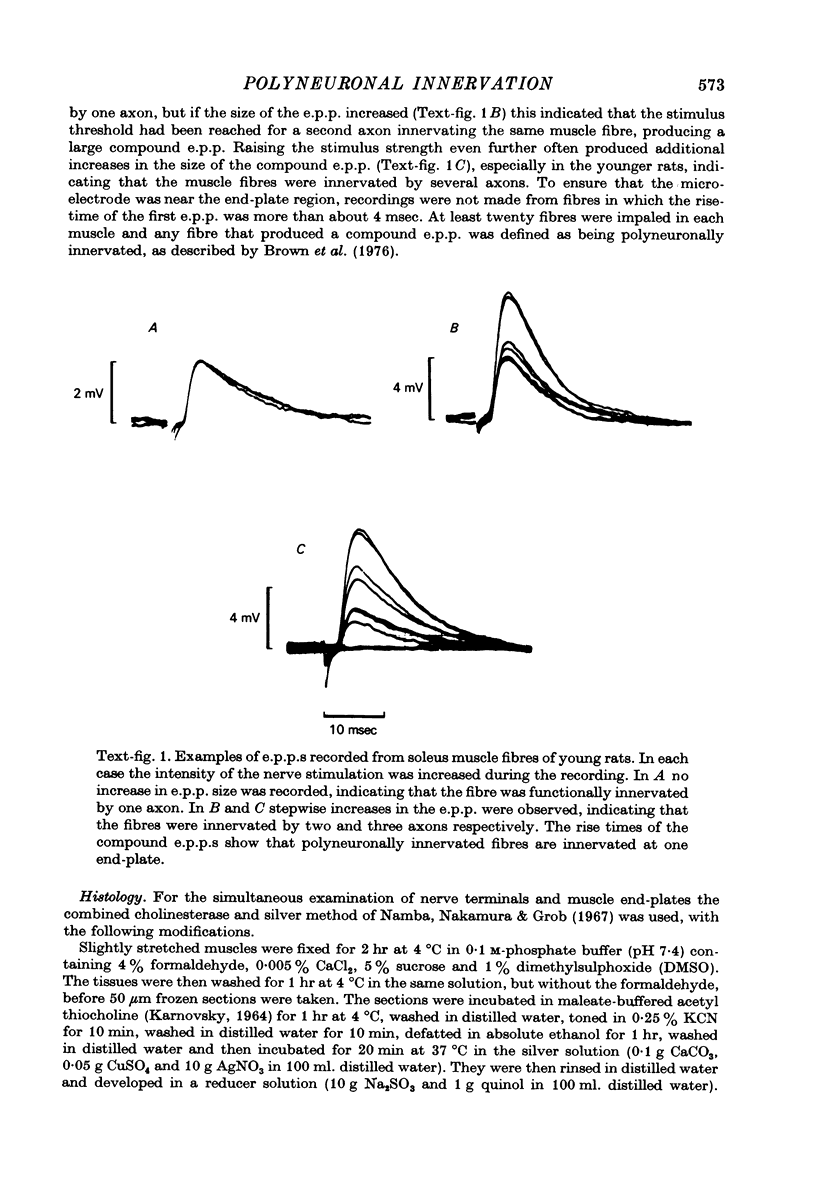
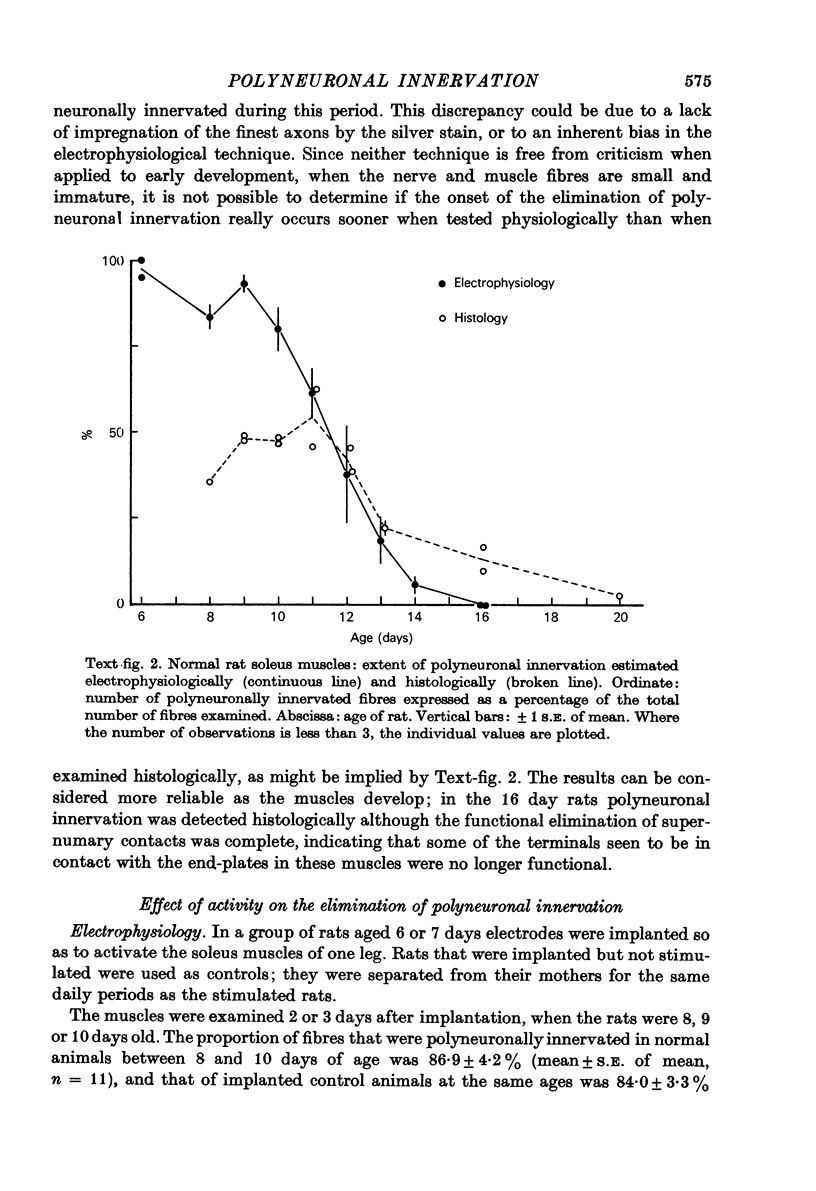
Abstract
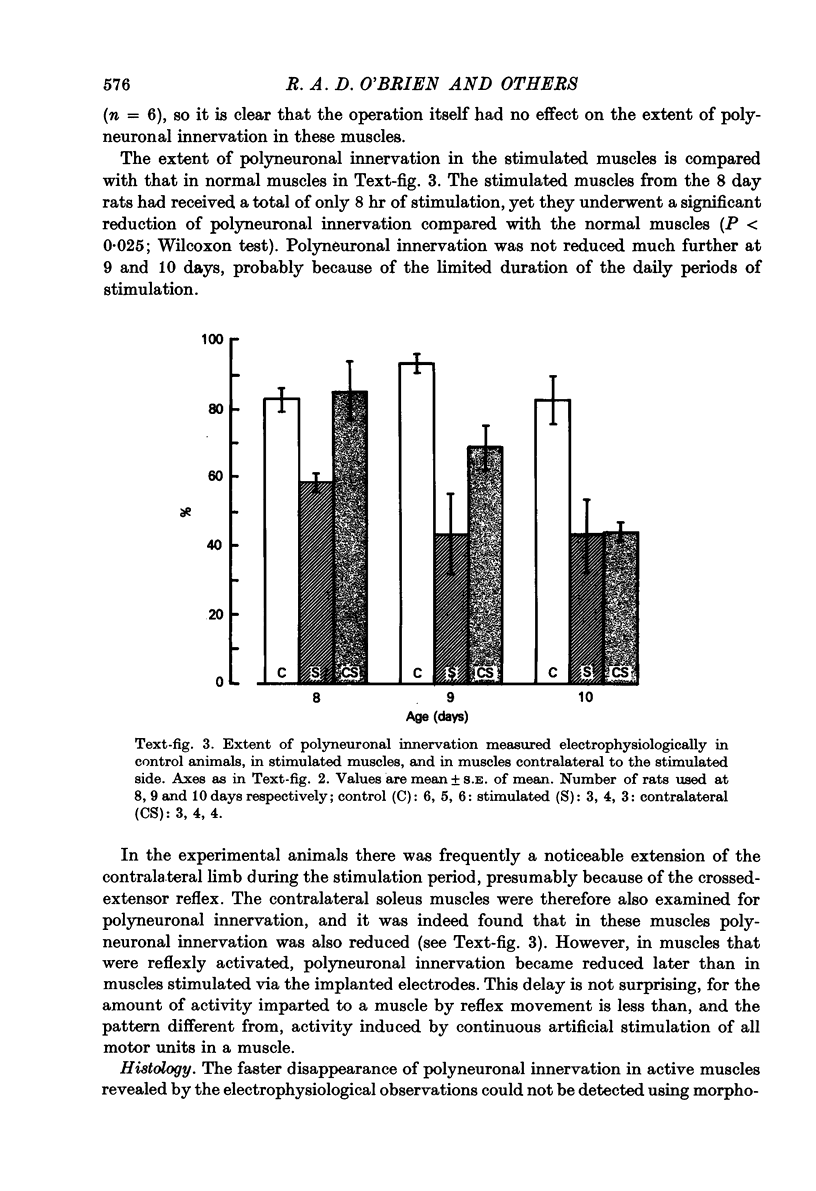
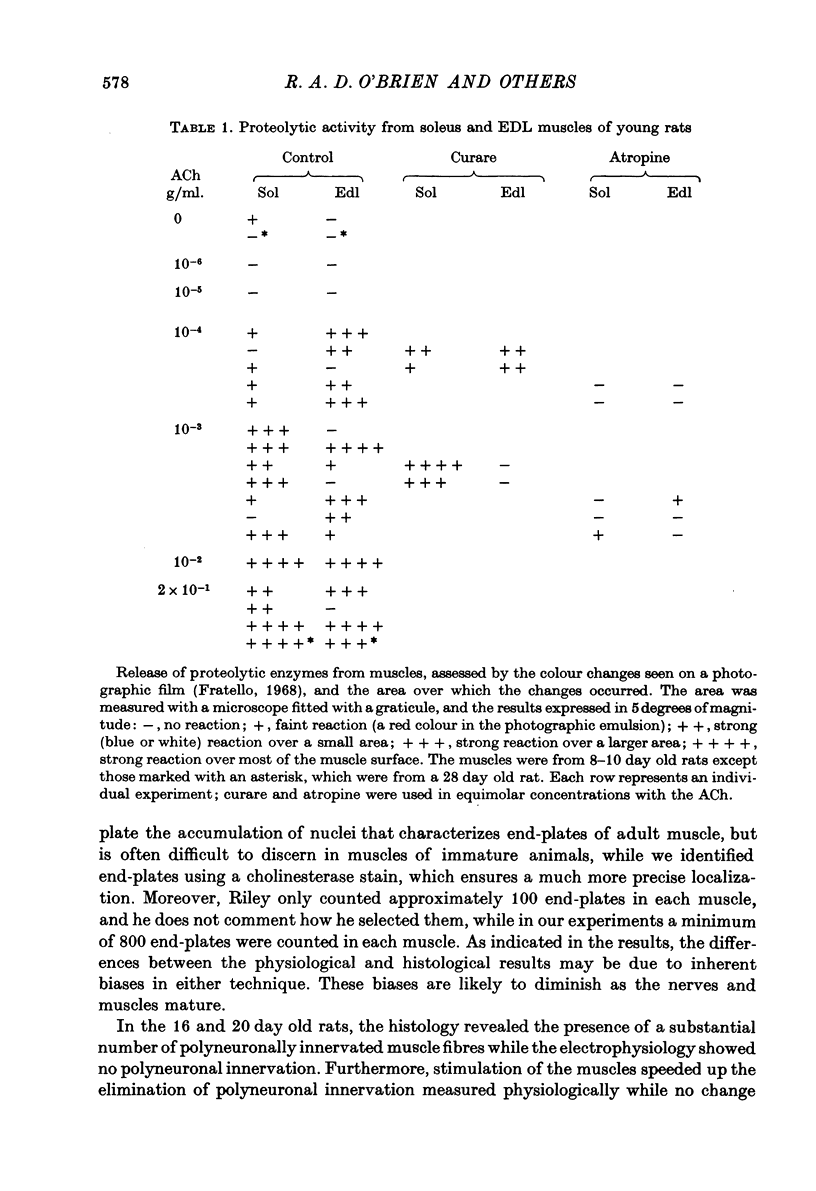
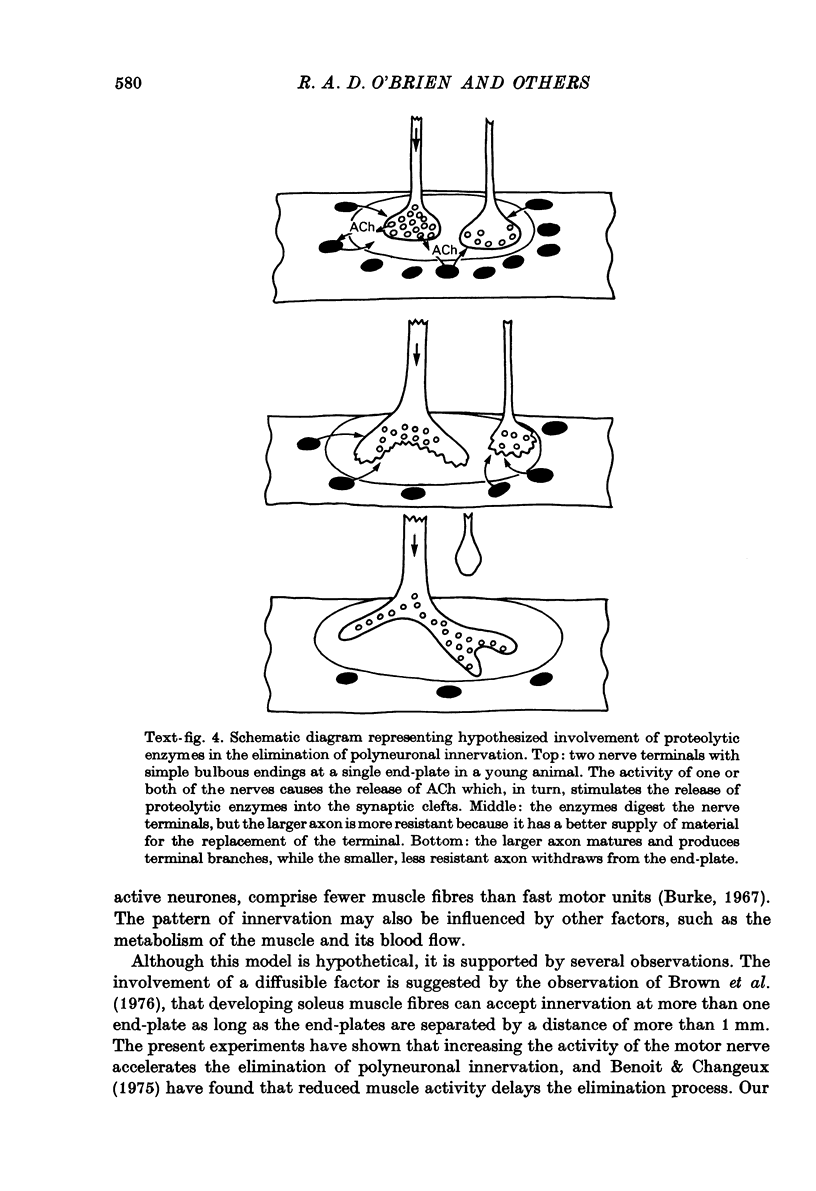
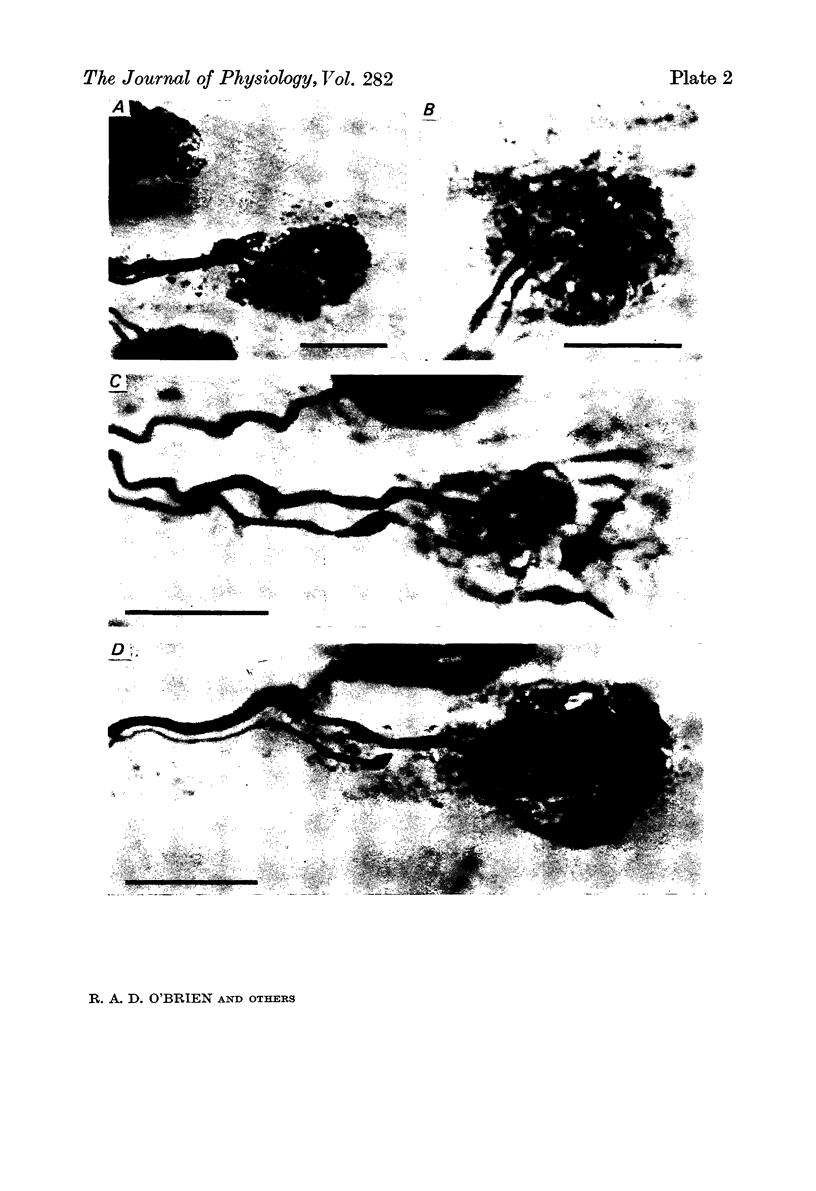
1. The mechanism responsible for the elimination of polyneuronal innervation in developing rat soleus muscles was studied electrophysiologically and histologically. 2. Initially all the axons contacting a single end-plate have simple bulbous terminals. As elimination proceeds one axon develops terminal branches while the other terminals remain bulbous and may be seen in contact with, or a short distance away from, the end-plate. It is suggested that the branched terminal remains in contact with the muscle fibre while the other terminals withdraw. 3. At a time when polyneuronal innervation can no longer be detected electrophysiologically, the histological technique still shows the presence of end-plates contacted by more than one nerve terminal. 4. The effect of activity on the disappearance of polyneuronal innervation was examined. Activity was increased by electrical stimulation of the right sciatic nerve. This procedure also produced reflex activity in the contralateral limb. In both cases polyneuronal innervation was eliminated more rapidly in the active muscles. 5. The finding that proteolytic enzymes are released from muscles treated with acetylcholine (ACh), and the observation of the more rapid elimination of supernumerary terminals at the end-plates of active muscles, lead to the suggestion that superfluous nerve-muscle contacts are removed by the proteolytic enzymes in response to neuromuscular activity. The selective stabilization of only one of the terminals is discussed in the light of these results.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagust J., Lewis D. M., Westerman R. A. Polyneuronal innervation of kitten skeletal muscle. J Physiol. 1973 Feb;229(1):241–255. doi: 10.1113/jphysiol.1973.sp010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in striated muscle during development. J Physiol. 1974 Sep;241(2):515–545. doi: 10.1113/jphysiol.1974.sp010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit P., Changeux J. P. Consequences of tenotomy on the evolution of multiinnervation in developing rat soleus muscle. Brain Res. 1975 Dec 5;99(2):354–358. doi: 10.1016/0006-8993(75)90036-0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J., MILEDI R. A study of foetal and new-born rat muscle fibres. J Physiol. 1962 Aug;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E., Jansen J. K., Lomo T., Westgaard R. H. The interaction between foreign and original motor nerves innervating the soleus muscle of rats. J Physiol. 1975 Jun;247(3):725–743. doi: 10.1113/jphysiol.1975.sp010954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratello B. Enhanced interpretation of tissue protease activity by use of photographic color film as a substrate. Stain Technol. 1968 May;43(3):125–128. doi: 10.3109/10520296809115054. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J. THE LOCALIZATION OF CHOLINESTERASE ACTIVITY IN RAT CARDIAC MUSCLE BY ELECTRON MICROSCOPY. J Cell Biol. 1964 Nov;23:217–232. doi: 10.1083/jcb.23.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneliussen H., Jansen J. K. Morphological aspects of the elimination of polyneuronal innervation of skeletal muscle fibres in newborn rats. J Neurocytol. 1976 Oct;5(8):591–604. doi: 10.1007/BF01175572. [DOI] [PubMed] [Google Scholar]
- Namba T., Nakamura T., Grob D. Staining for nerve fiber and cholinesterase activity in fresh frozen sections. Am J Clin Pathol. 1967 Jan;47(1):74–77. doi: 10.1093/ajcp/47.1.74. [DOI] [PubMed] [Google Scholar]
- O'Brien R. A., Purves R. D., Vrbová G. Effect of activity on the elimination of multiple innervation in soleus muscles of rats [proceedings]. J Physiol. 1977 Oct;271(2):54P–55P. [PubMed] [Google Scholar]
- Poberai M., Sávay G., Csillik B. Function-dependent proteinase activity in the neuromuscular synapse. Neurobiology. 1972;2(1):1–7. [PubMed] [Google Scholar]
- Poberai M., Sávay G. Time course of proteolytic enzyme alterations in the motor end-plates after stimulation. Acta Histochem. 1976;57(1):44–48. doi: 10.1016/S0065-1281(76)80006-2. [DOI] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley D. A. Spontaneous elimination of nerve terminals from the endplates of developing skeletal myofibers. Brain Res. 1977 Oct 7;134(2):279–285. doi: 10.1016/0006-8993(77)91073-3. [DOI] [PubMed] [Google Scholar]