Abstract
1. The relationship between adrenalcortical hormones, adrenocorticotrophic hormone (ACTH), and pain sensitivity was investigated in the rat. Pain sensitivity was assessed by measuring paw-lick and jump latencies in response to being placed on a grid at 55 °C.
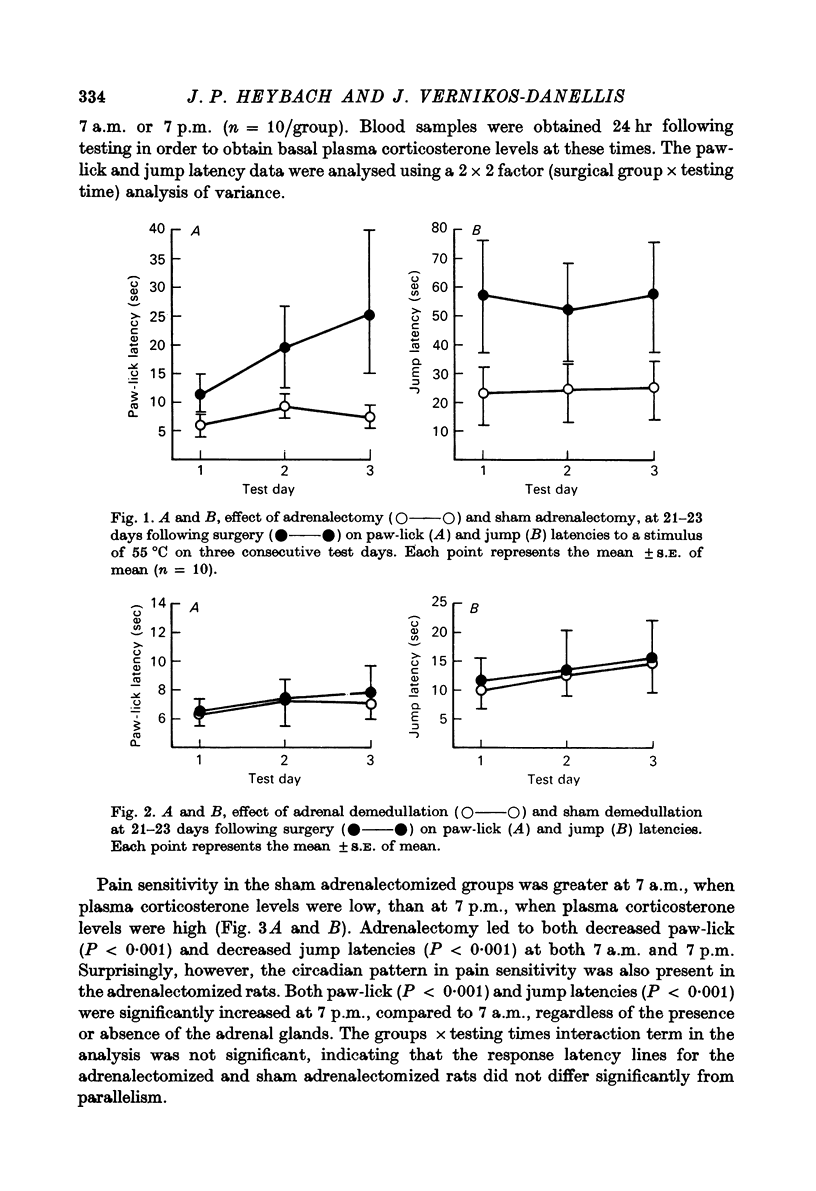
2. Bilateral adrenalectomy increased the sensitivity to pain, but adrenal demedullation had no effect.
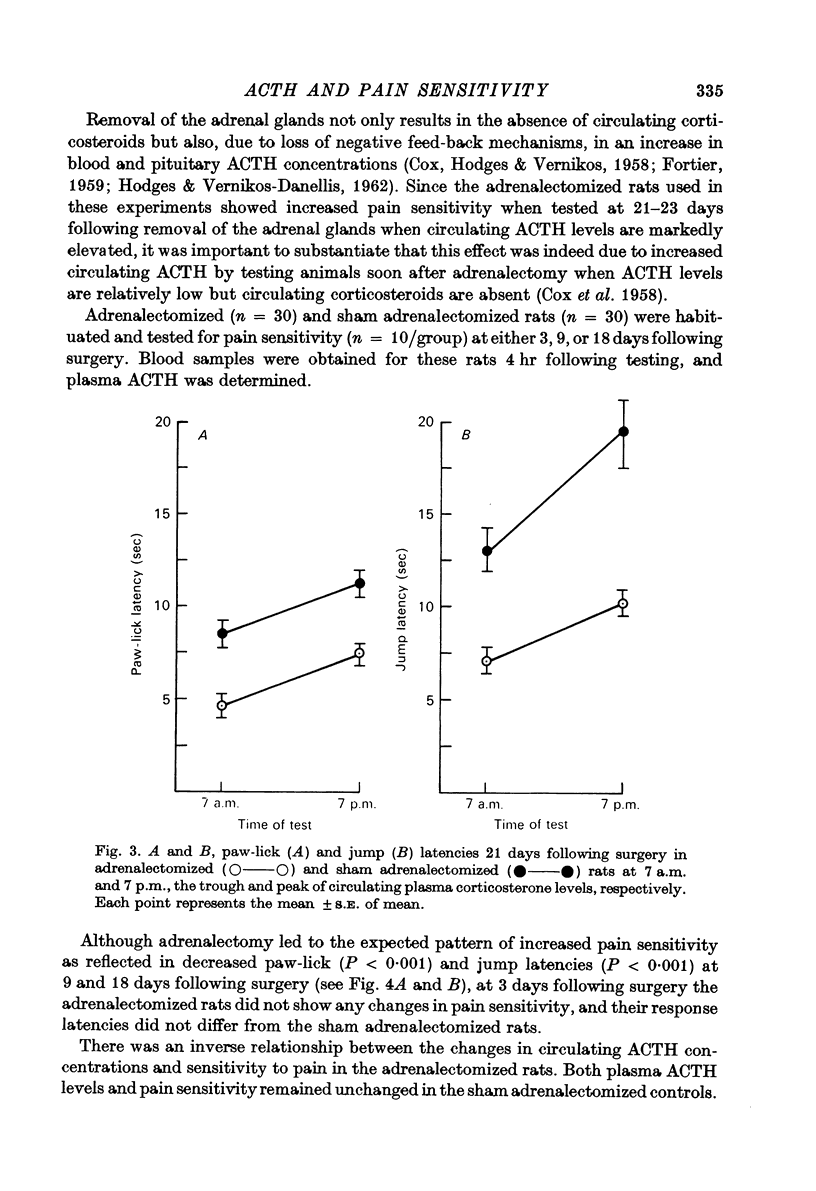
3. Pain sensitivity was inversely related to the circadian changes in circulating corticosterone and was greater at 7 a.m. than at 7 p.m. However, the same variation in pain sensitivity existed if the adrenals were removed, suggesting that the increase in pain sensitivity after adrenalectomy was not related directly to the levels of corticosteriods.
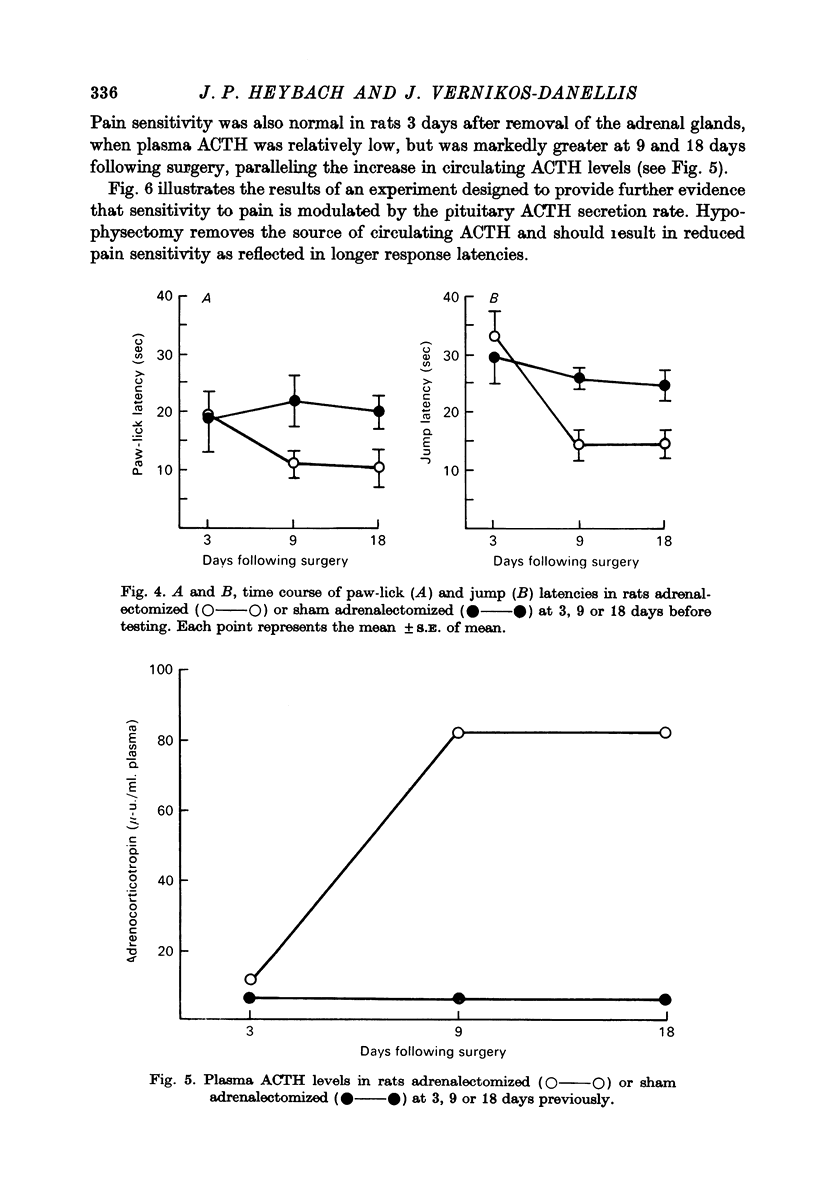
4. The time course of the increase in pain sensitivity after adrenalectomy paralleled that of the changes in circulating ACTH. Adrenalectomy markedly increased pain sensitivity at 9 and 18 days following surgery when circulating ACTH levels were markedly elevated and corticosterone was absent, but not at 3 days following adrenalectomy when ACTH levels were lower and corticosterone was absent.
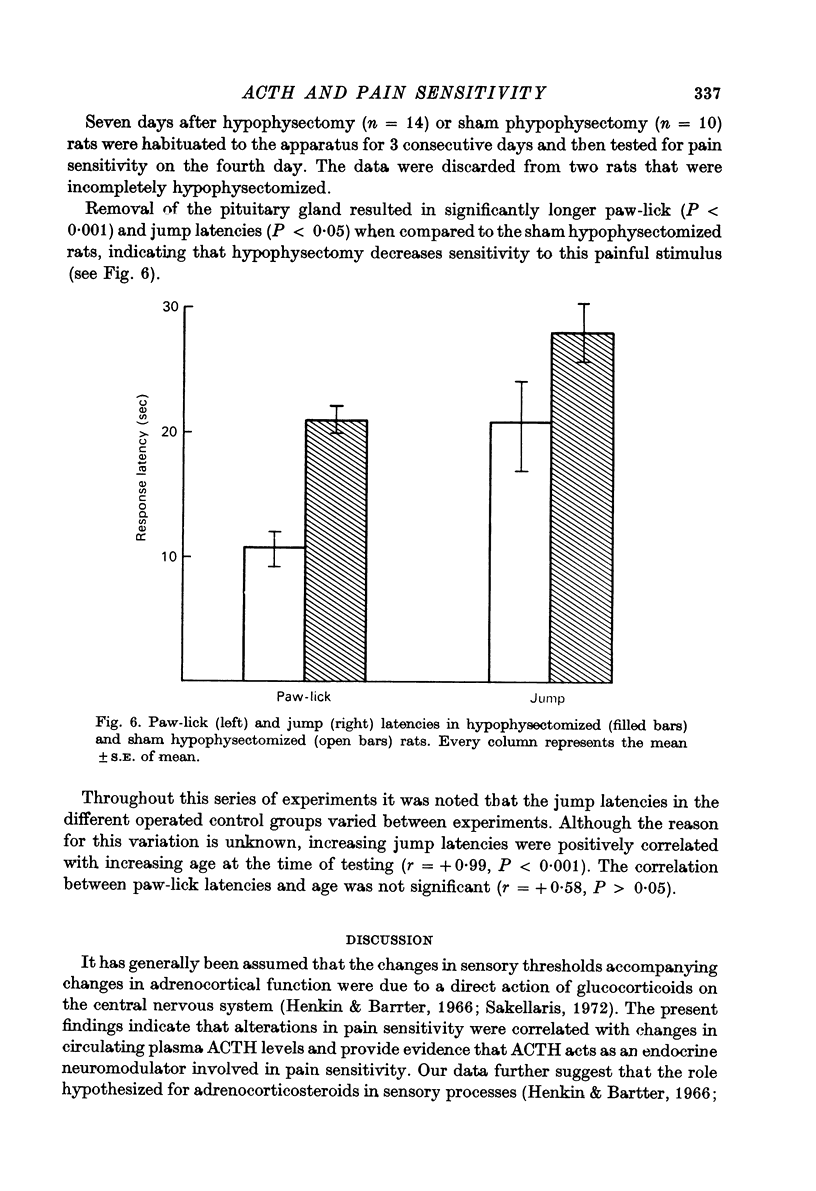
5. Hypophysectomy decreased the sensitivity to pain.
6. The results indicate that ACTH can alter pain sensitivity and that the effect of corticosteroids on the sensitivity to pain is an indirect one by virtue of their negative feed-back action on the hypothalamic-pituitary system.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Nicholson W. E., Liddle G. W., Orth D. N., Island D. P. Normal and abnormal regulation of beta-msh in man. J Clin Invest. 1969 Aug;48(8):1580–1585. doi: 10.1172/JCI106123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein M. J., Mroz E. A., Kizer J. S., Palkovits M., Leeman S. E. Regional distribution of substance P in the brain of the rat. Brain Res. 1976 Nov 5;116(2):299–305. doi: 10.1016/0006-8993(76)90907-0. [DOI] [PubMed] [Google Scholar]
- Buckingham J. C., Hodges J. R. Interrelationships of pituitary and plasma corticotrophin and plasma corticosterone during adrenocortical regeneration in the rat. J Endocrinol. 1975 Dec;67(3):411–417. doi: 10.1677/joe.0.0670411. [DOI] [PubMed] [Google Scholar]
- COX G. S., HODGES J. R., VERNIKOS J. The effect of adrenalectomy on the circulating level of adrenocorticotrophic hormone in the rat. J Endocrinol. 1958 Jul;17(2):177–181. doi: 10.1677/joe.0.0170177. [DOI] [PubMed] [Google Scholar]
- FORTIER C. Pituitary ACTH and plasma free corticosteroids following bilateral adrenalectomy in the rat. Proc Soc Exp Biol Med. 1959 Jan;100(1):13–16. doi: 10.3181/00379727-100-24506. [DOI] [PubMed] [Google Scholar]
- Gay V. L. A stereotaxic approach to transauricular hypophysectomy in the rat. Endocrinology. 1967 Nov;81(5):1177–1179. doi: 10.1210/endo-81-5-1177. [DOI] [PubMed] [Google Scholar]
- Gibbs J., Sechzer J. A., Smith G. P. Behavioral responsiveness of adrenalectomized, hypophysectomized, and intact rats to electric shock. J Comp Physiol Psychol. 1973 Jan;82(1):165–169. doi: 10.1037/h0033796. [DOI] [PubMed] [Google Scholar]
- Gilkes J. J., Bloomfield G. A., Scott A. P., Lowry P. J., Ratcliffe J. G., Landon J., Rees L. H. Development and validation of a radioimmunoassay for peptides related to beta-melanocyte-stimulating hormone in human plasma: the lipotropins. J Clin Endocrinol Metab. 1975 Mar;40(3):450–457. doi: 10.1210/jcem-40-3-450. [DOI] [PubMed] [Google Scholar]
- Gispen W. H., Buitelaar J., Wiegant V. M., Terenius L., De Wied D. Interaction between ACTH fragments, brain opiate receptors and morphine-induced analgesia. Eur J Pharmacol. 1976 Oct;39(2):393–397. doi: 10.1016/0014-2999(76)90150-3. [DOI] [PubMed] [Google Scholar]
- Goldstein A. Opioid peptides endorphins in pituitary and brain. Science. 1976 Sep 17;193(4258):1081–1086. doi: 10.1126/science.959823. [DOI] [PubMed] [Google Scholar]
- Greven H. M., de Wied D. The influence of peptides derived from corticotrophin (ACTH) on performance. Structure activity studies. Prog Brain Res. 1973;39:429–442. doi: 10.1016/S0079-6123(08)64098-4. [DOI] [PubMed] [Google Scholar]
- Guillemin R., Vargo T., Rossier J., Minick S., Ling N., Rivier C., Vale W., Bloom F. beta-Endorphin and adrenocorticotropin are selected concomitantly by the pituitary gland. Science. 1977 Sep 30;197(4311):1367–1369. doi: 10.1126/science.197601. [DOI] [PubMed] [Google Scholar]
- HODGES J. R., VERNIKOS-DANELLIS J. Pituitary and blood corticotrophin changes in adrenalectomized rats maintained on physiological doses of corticosteroids. Acta Endocrinol (Copenh) 1962 Jan;39:79–86. doi: 10.1530/acta.0.0390079. [DOI] [PubMed] [Google Scholar]
- HODGES J. R., VERNIKOS J. Circulating corticotrophin in normal and adrenalectomized rats after stress. Acta Endocrinol (Copenh) 1959 Feb;30(2):188–196. doi: 10.1530/acta.0.0300188. [DOI] [PubMed] [Google Scholar]
- HODGES J. R., VERNIKOS J. The effects of hydrocortisone on the level of corticotrophin in the blood and pituitary glands of adrenalectomized and of stressed adrenalectomized rats. J Physiol. 1960 Mar;150:683–693. doi: 10.1113/jphysiol.1960.sp006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin R. I., Bartter F. C. Studies on olfactory thresholds in normal man and in patients with adrenal cortical insufficiency: the role of adrenal cortical steroids and of serum sodium concentration. J Clin Invest. 1966 Oct;45(10):1631–1639. doi: 10.1172/JCI105470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin R. I., McGlone R. E., Daly R., Bartter F. C. Studies on auditory thresholds in normal man and in patients with adrenal cortical insufficiency: the role of adrenal cortical steroids. J Clin Invest. 1967 Mar;46(3):429–435. doi: 10.1172/JCI105544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosowicz J., Pruszewicz A. The "taste" test in adrenal insufficiency. J Clin Endocrinol Metab. 1967 Feb;27(2):214–218. doi: 10.1210/jcem-27-2-214. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Allen W., Rizzo F., Krieger H. P. Characterization of the normal temporal pattern of plasma corticosteroid levels. J Clin Endocrinol Metab. 1971 Feb;32(2):266–284. doi: 10.1210/jcem-32-2-266. [DOI] [PubMed] [Google Scholar]
- LUFT R., OLIVECRONA H. Hypophysectomy in man; experiences in metastatic cancer of the breast. Cancer. 1955 Mar-Apr;8(2):261–270. doi: 10.1002/1097-0142(1955)8:2<261::aid-cncr2820080205>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A., Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3014–3018. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier G., Leclerc R., Labrie F., Cote J., Chretien M., Lis M. Immunohistochemical localization of beta-lipotropic hormone in the pituitary gland. Endocrinology. 1977 Mar;100(3):770–776. doi: 10.1210/endo-100-3-770. [DOI] [PubMed] [Google Scholar]
- SYDNOR K. L., SAYERS G. Blood and pituitary ACTH in intact and adrenalectomized rats after stress. Endocrinology. 1954 Nov;55(5):621–636. doi: 10.1210/endo-55-5-621. [DOI] [PubMed] [Google Scholar]
- Sakellaris P. C. Olfactory thresholds in normal and adrenalectomized rats. Physiol Behav. 1972 Oct;9(4):495–500. doi: 10.1016/0031-9384(72)90003-0. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Konishi S., Powell D., Leeman S. E., Otsuka M. Identification of the motoneuron-depolarizing peptide in bovine dorsal root as hypothalamic substance P. Brain Res. 1974 Jun 14;73(1):59–69. doi: 10.1016/0006-8993(74)91007-5. [DOI] [PubMed] [Google Scholar]
- Terenius L. Somatostatin and ACTH are peptides with partial antagonist-like selectivity for opiate receptors. Eur J Pharmacol. 1976 Jul;38(1):211–213. doi: 10.1016/0014-2999(76)90221-1. [DOI] [PubMed] [Google Scholar]
- Tseng L. F., Loh H. H., Li C. H. Beta-Endorphin as a potent analgesic by intravenous injection. Nature. 1976 Sep 16;263(5574):239–240. doi: 10.1038/263239a0. [DOI] [PubMed] [Google Scholar]
- VERNIKOS-DANELLIS J. EFFECT OF STRESS, ADRENALECTOMY, HYPOPHYSECTOMY AND HYDROCORTISONE ON THE CORTICOTROPIN-RELEASING ACTIVITY OF RAT MEDIAN EMINENCE. Endocrinology. 1965 Jan;76:122–126. doi: 10.1210/endo-76-1-122. [DOI] [PubMed] [Google Scholar]
- Vernikos-Danellis J., Anderson E., Trigg L. Changes in adrenal corticosterone concentration in rats: method of bio-assay for ACTH. Endocrinology. 1966 Sep;79(3):624–630. doi: 10.1210/endo-79-3-624. [DOI] [PubMed] [Google Scholar]
- de Wied D. Inhibitory effect of ACTH and related peptides on extinction of conditioned avoidance behavior in rats. Proc Soc Exp Biol Med. 1966 May;122(1):28–32. doi: 10.3181/00379727-122-31042. [DOI] [PubMed] [Google Scholar]


