Abstract
1. The membrane characteristics of the slowly adapting stretch receptor from the crayfish, Astacus fluviatilis, were examined with electrophysiological techniques consisting of membrane potential recording, voltage clamp and ion-sensitive microelectrodes. 2. The passive membrane current (Ip) following step changes of the membrane potential to levels above 0 mV required more than a minute to decay to a steady-state level. 3. The stretch-induced current (SIC, where SIC = Itotal--Ipassive) was not fully developed until the Ip had decayed to a steady state. 4. With Ip at the steady state and the stretch-induced current at the O-current potential, a slow stretch-induced inward current was isolated. The latter reaches a maximum after 1 sec of stretch and declines even more slowly after stretch. The I-V relation of the slow current had a negative slope and reversed sign near the resting potential. It is suggested that this current is due to a Cl- conductance change. 5. The stretch-induced current, consisting of a rapid transient phase and a steady component can be isolated from the slow stretch-induced current at a holding potential corresponding to the resting potential. 6. The SIC-Em relation is non-linear and reverses sign at about +15 mV. 7. In a given cell, the reversal potential of the stretch-induced potential change obtained with current clamp coincided with the 0-current potential of the stretch-induced current obtained by voltage clamp. The average value from twenty-six cells was +13 +/- 6.5 mV; cell to cell variability seemed to be correlated with dendrite length. 8. Tris (mol. wt. 121) or arginine (mol. wt. 174) susbstituted for Na+ reduces but does not abolish the stretch-induced current. 9. The permeability ratios of Tris:Na and arginine:Na were estimated from changes in the 0-current potential as these cations replaced Na+ in the external medium. The PTris:PNa was somewhat higher (0.31) than the Parginine:PNa ratio (0.25). 10. Changes in the external Ca2+ concentration had no effect on the 0-current potential in Na or Tris saline. However, reducing Ca2+ did augment the stretch-induced current in either saline. A tenfold reduction of Ca2+ increased the conductance (at the 0-current level) about twofold. 11. Intracellular K+ and Cl- activities were obtained with ion sensitive electrodes. The average values from six cells were aiK = 133 +/- 34 mM and aiCl = 15.2 +/- 1.8 mM S.D.). EK was about 20 mV more negative than Em and ECl was about 10 mV more positive than Em. 12. aik and resting Em undergo large changes in K+-free solutions. After 60 min, ak was reduced eightfold and Em was reduced from -67 to -40 mV. Reduced Ca2+ in K+-free augments the rate of these changes. Receptor potential amplitude was also reduced in K+-free solution but could be restored upon polarizing the membrane to the pre-existing resting level.
Full text
PDF

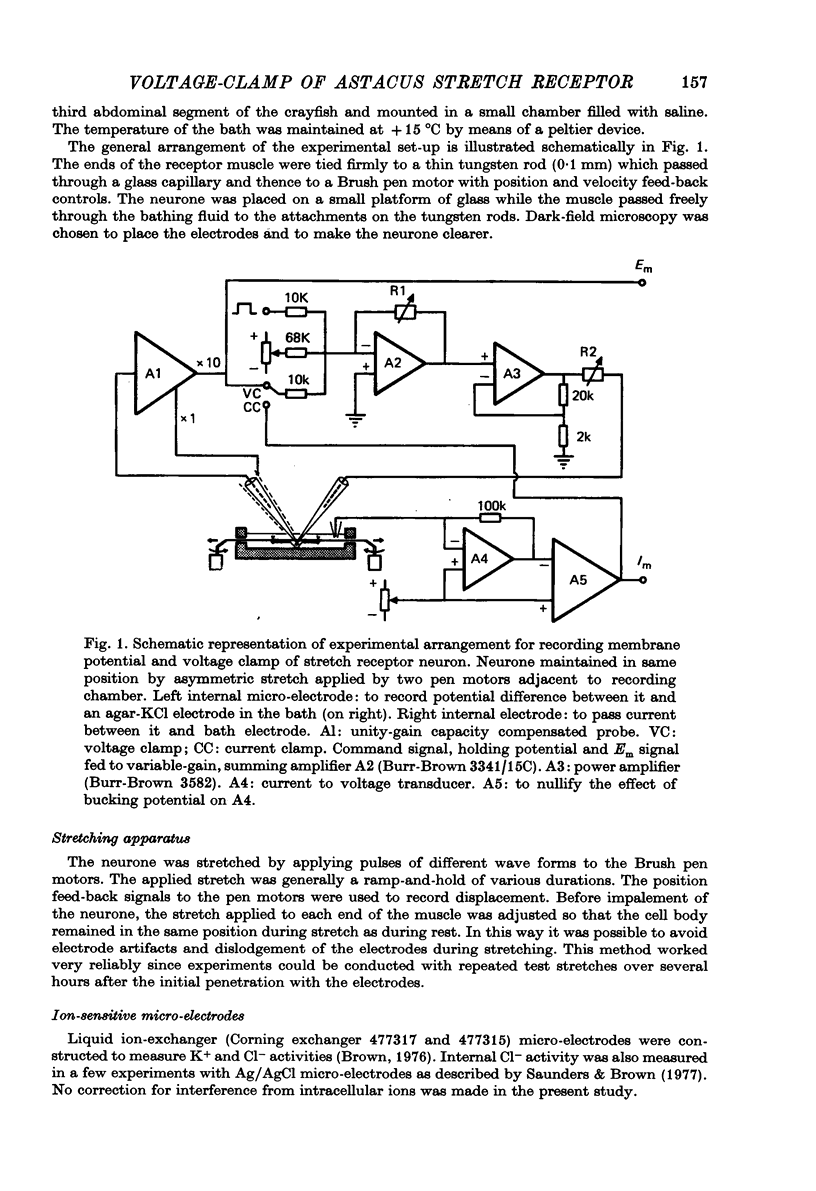
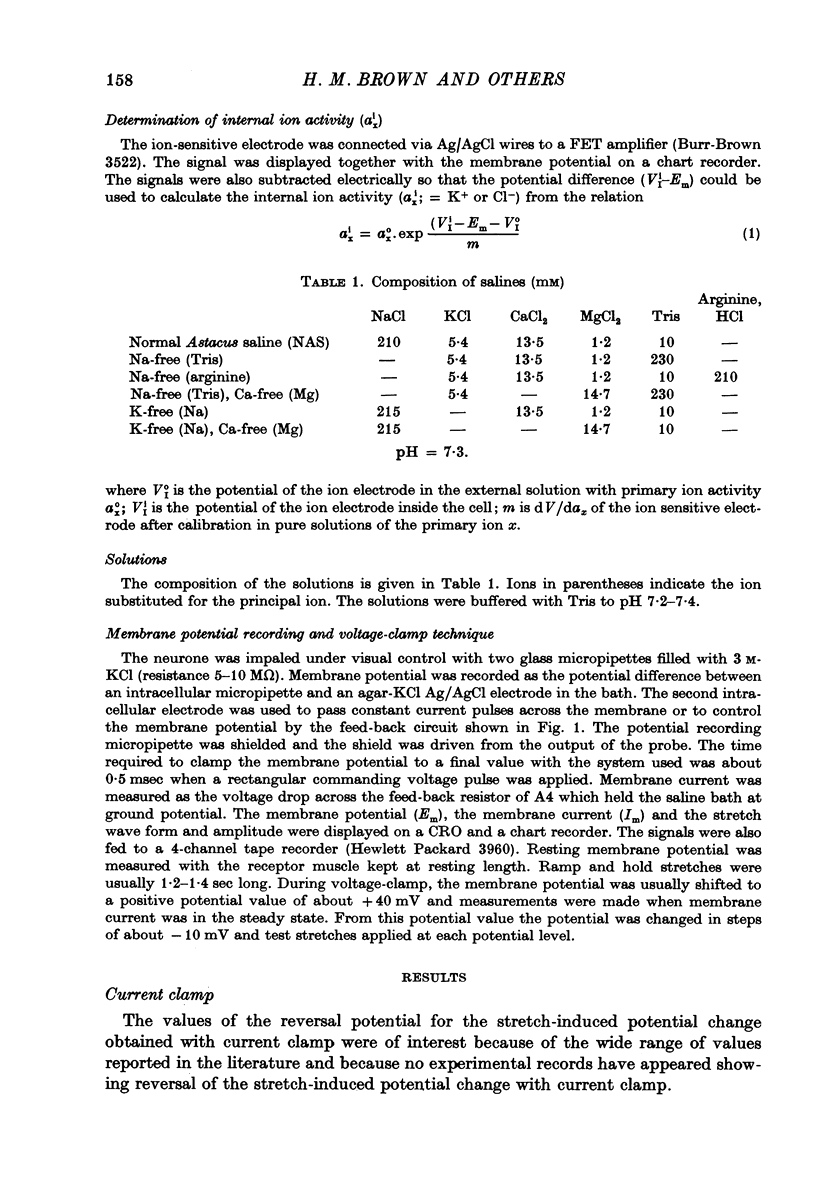
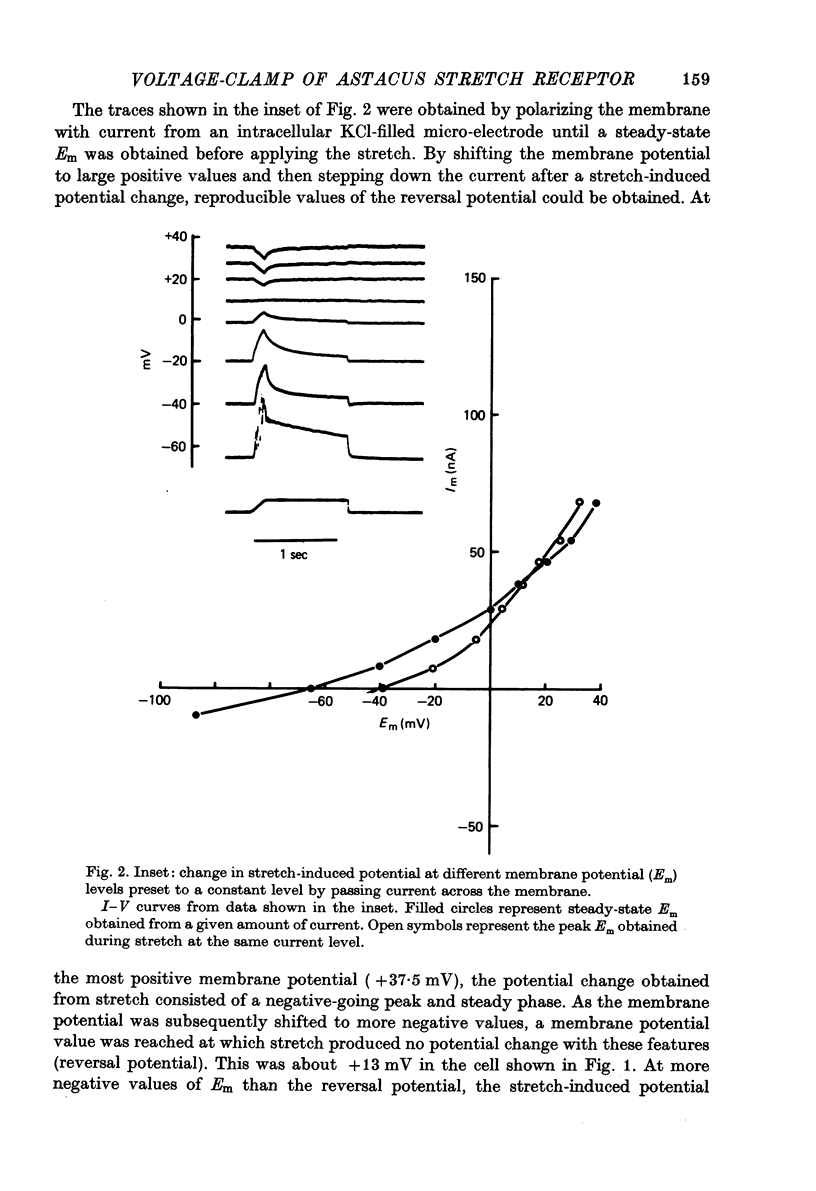
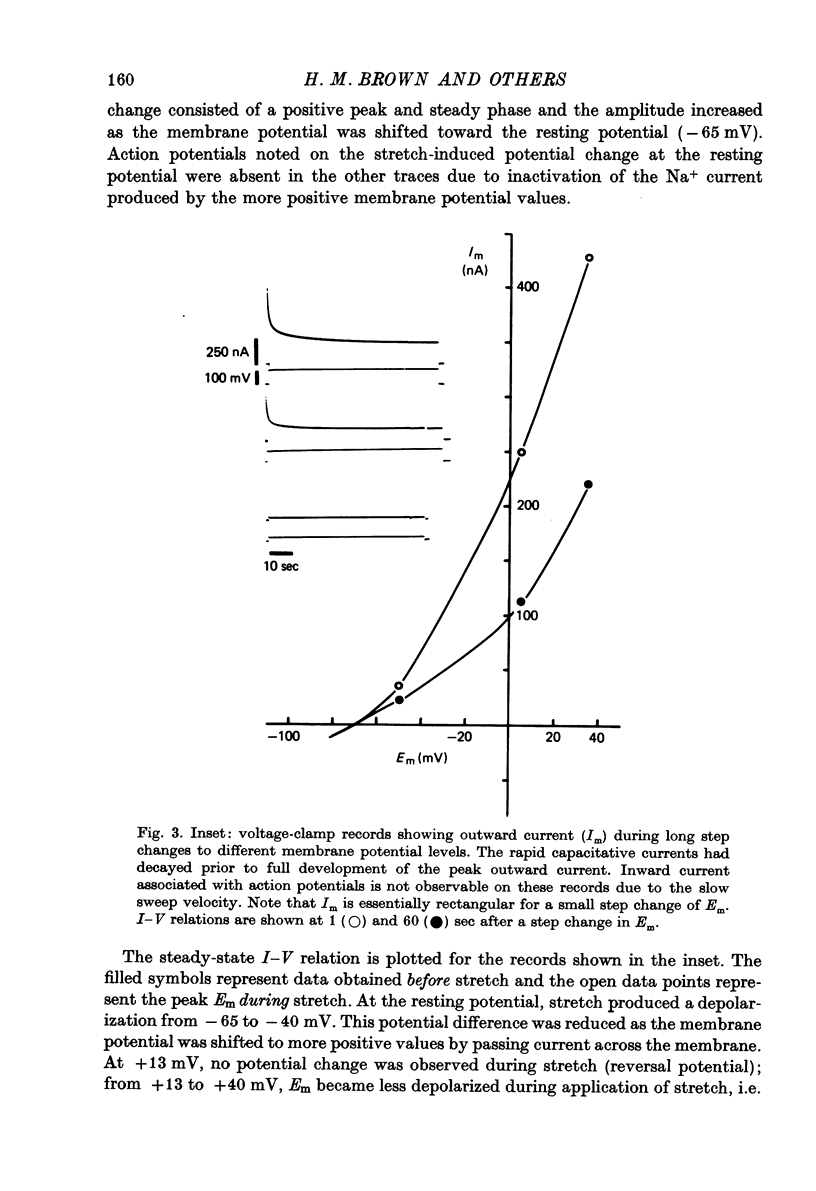


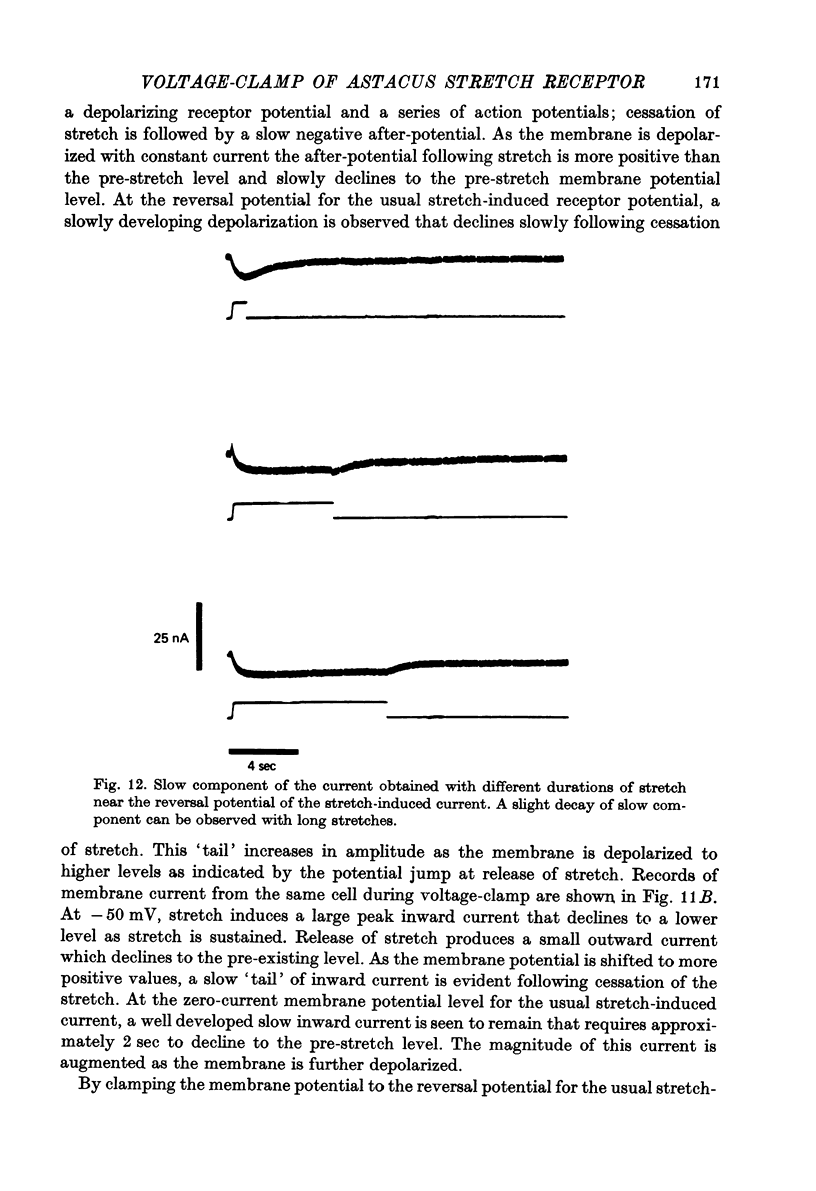
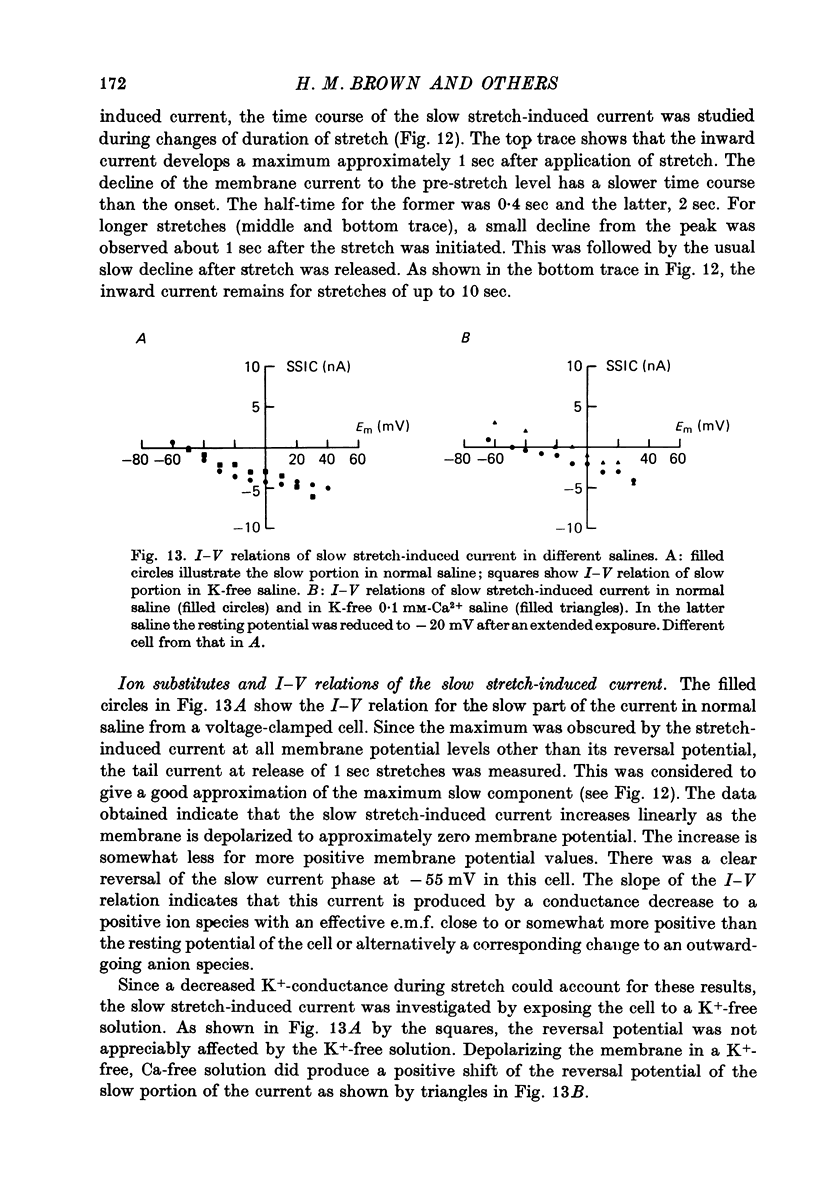
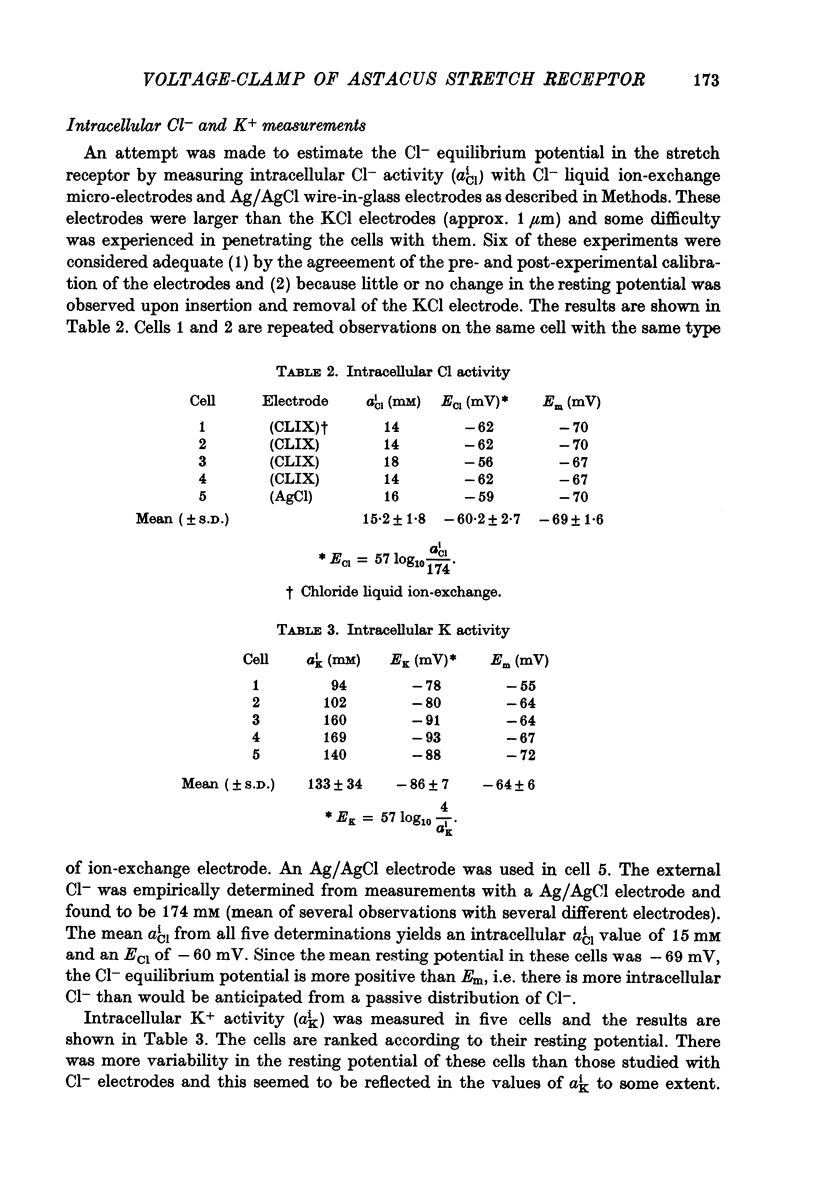
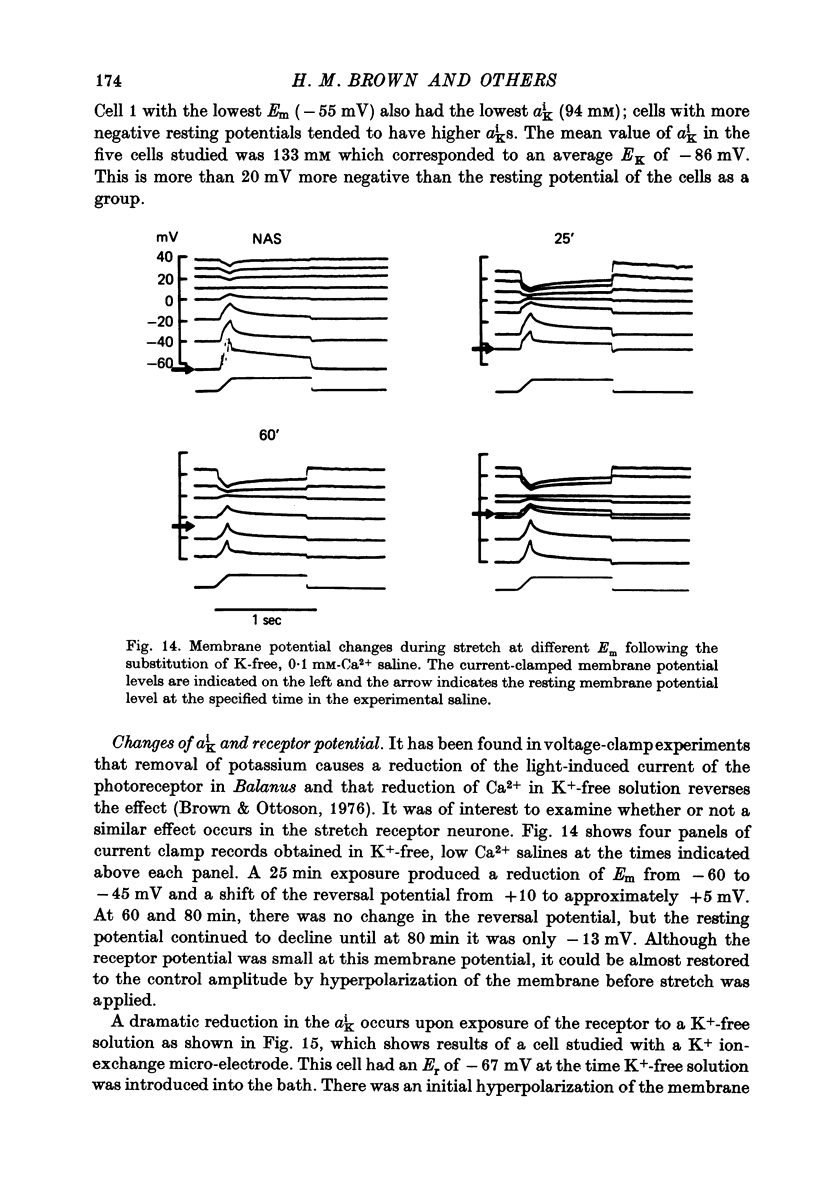




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown H. M., Hagiwara S., Koike H., Meech R. M. Membrane properties of a barnacle photoreceptor examined by the voltage clamp technique. J Physiol. 1970 Jun;208(2):385–413. doi: 10.1113/jphysiol.1970.sp009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M. Intracellular Na+, K+, and C1- activities in Balanus photoreceptors. J Gen Physiol. 1976 Sep;68(3):281–296. doi: 10.1085/jgp.68.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Ottoson D. Dual role for potassium in Balanus photoreceptor: antagonist of calcium and suppression of light-induced current. J Physiol. 1976 May;257(2):355–378. doi: 10.1113/jphysiol.1976.sp011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Saunders J. H. Cation and anion sequences in dark-adapted Balanus photoreceptor. J Gen Physiol. 1977 Oct;70(4):531–543. doi: 10.1085/jgp.70.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Mote M. I. Ionic dependence of reversal voltage of the light response in Limulus ventral photoreceptors. J Gen Physiol. 1974 Mar;63(3):337–350. doi: 10.1085/jgp.63.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall M. C., Peterson D. F., Kunze D. L., Walker J. L., Brown A. M. Intracellular potassium and chloride activities measured with liquid ion exchanger microelectrodes. Brain Res. 1970 Oct 28;23(3):433–436. doi: 10.1016/0006-8993(70)90070-3. [DOI] [PubMed] [Google Scholar]
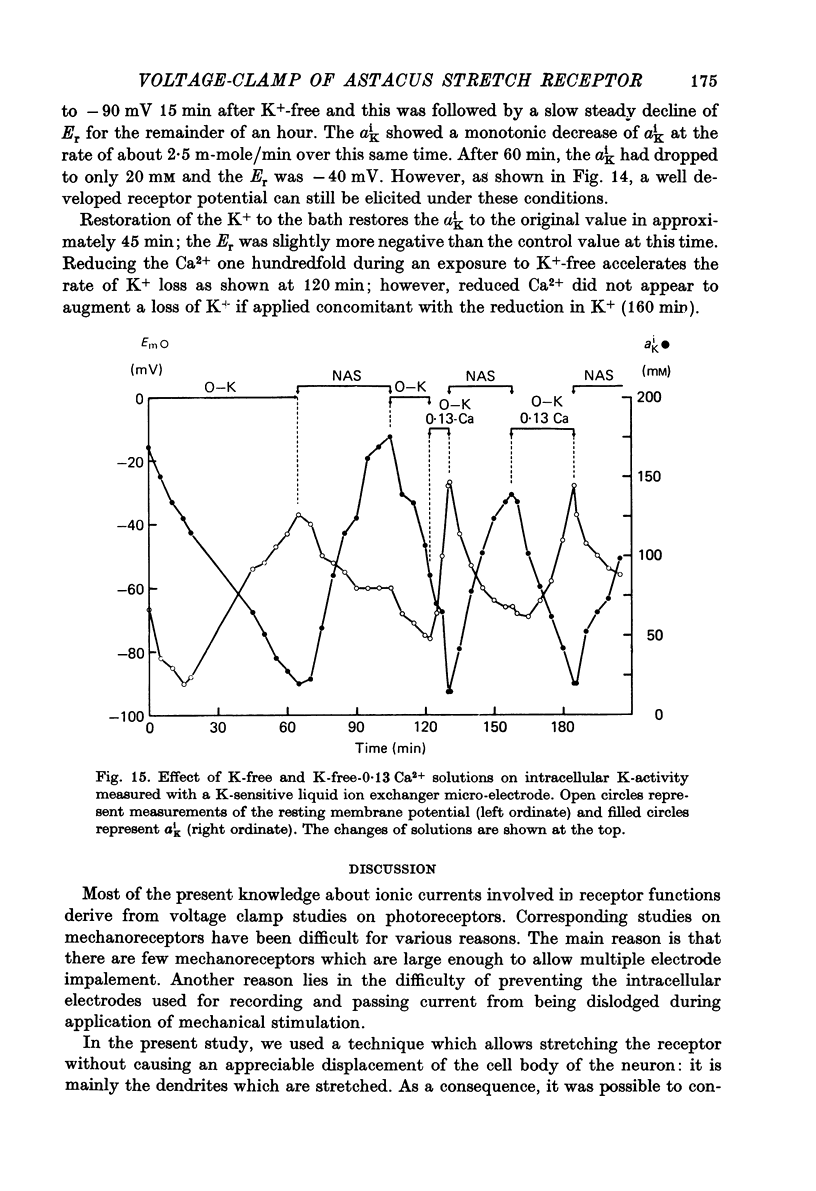
- DIAMOND J., GRAY J. A., INMAN D. R. The relation between receptor potentials and the concentration of sodium ions. J Physiol. 1958 Jul 14;142(2):382–394. doi: 10.1113/jphysiol.1958.sp006024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS C., HAGIWARA S. Potassium ions and the inhibitory process in the crayfish stretch receptor. J Gen Physiol. 1959 Nov;43:315–321. doi: 10.1085/jgp.43.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS C., TERZUOLO C. A., WASHIZU H. THE EFFECT OF CHANGES OF THE IONIC ENVIRONMENT UPON AN ISOLATED CRUSTACEAN SENSORY NEURON. J Neurophysiol. 1963 Nov;26:948–957. doi: 10.1152/jn.1963.26.6.948. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO S. Membrane changes in crayfish stretch receptor neutron during synaptic inhibition and under action of gamma-aminobutyric acid. J Neurophysiol. 1960 Sep;23:505–515. doi: 10.1152/jn.1960.23.5.505. [DOI] [PubMed] [Google Scholar]
- Husmark I., Ottoson D. Impulse activity of the isolated spindle in potassium-free solution. Acta Physiol Scand. 1971 Dec;83(4):486–494. doi: 10.1111/j.1748-1716.1971.tb05106.x. [DOI] [PubMed] [Google Scholar]
- Husmark I., Ottoson D. Is the adaptation of the muscle spindle of ionic origin? Acta Physiol Scand. 1971 Jan;81(1):138–140. doi: 10.1111/j.1748-1716.1971.tb04883.x. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., EYZAGUIRRE C. Synaptic inhibition in an isolated nerve cell. J Gen Physiol. 1955 Sep 20;39(1):155–184. doi: 10.1085/jgp.39.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
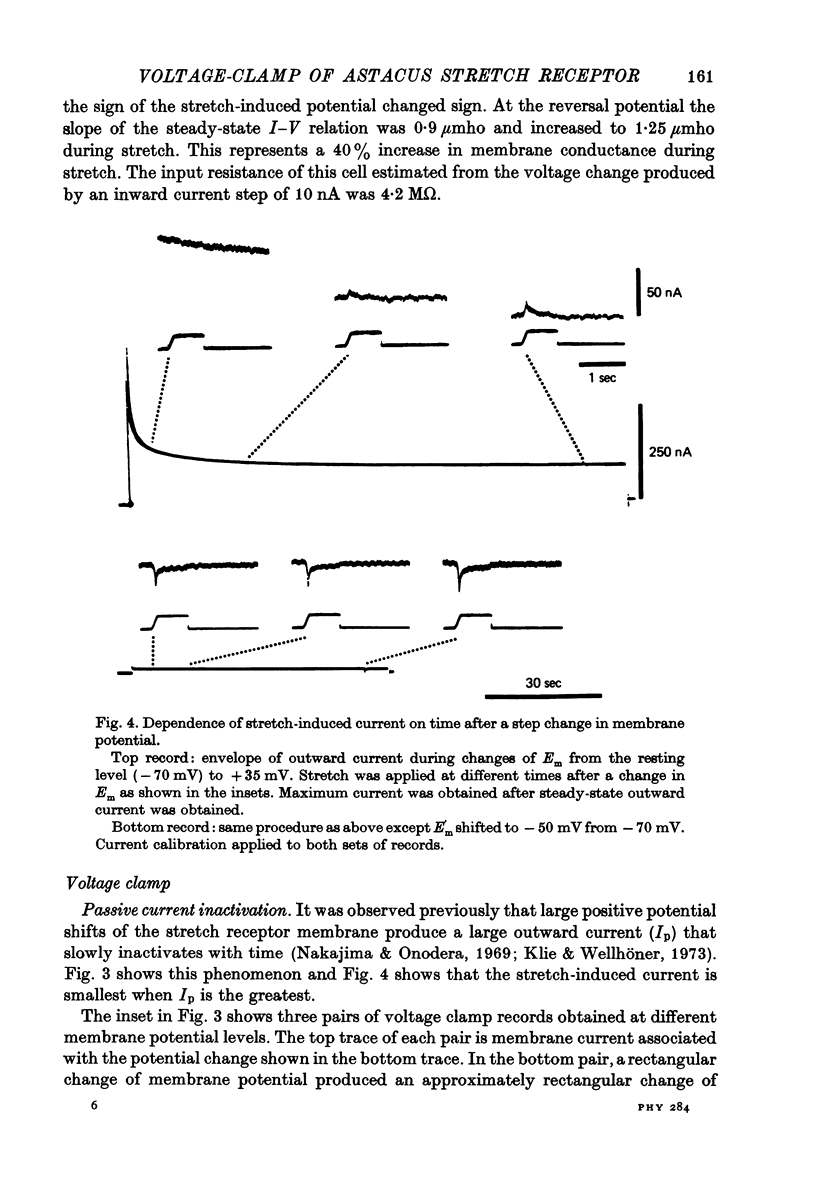
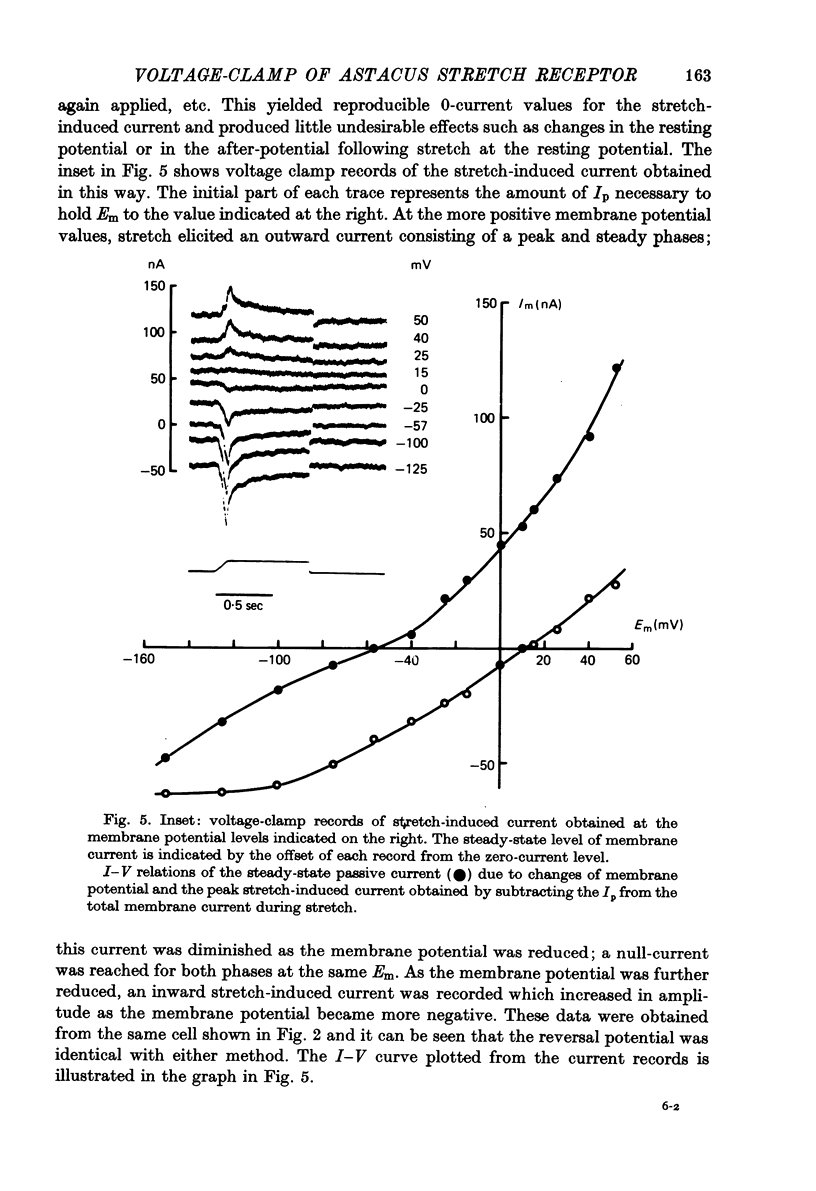
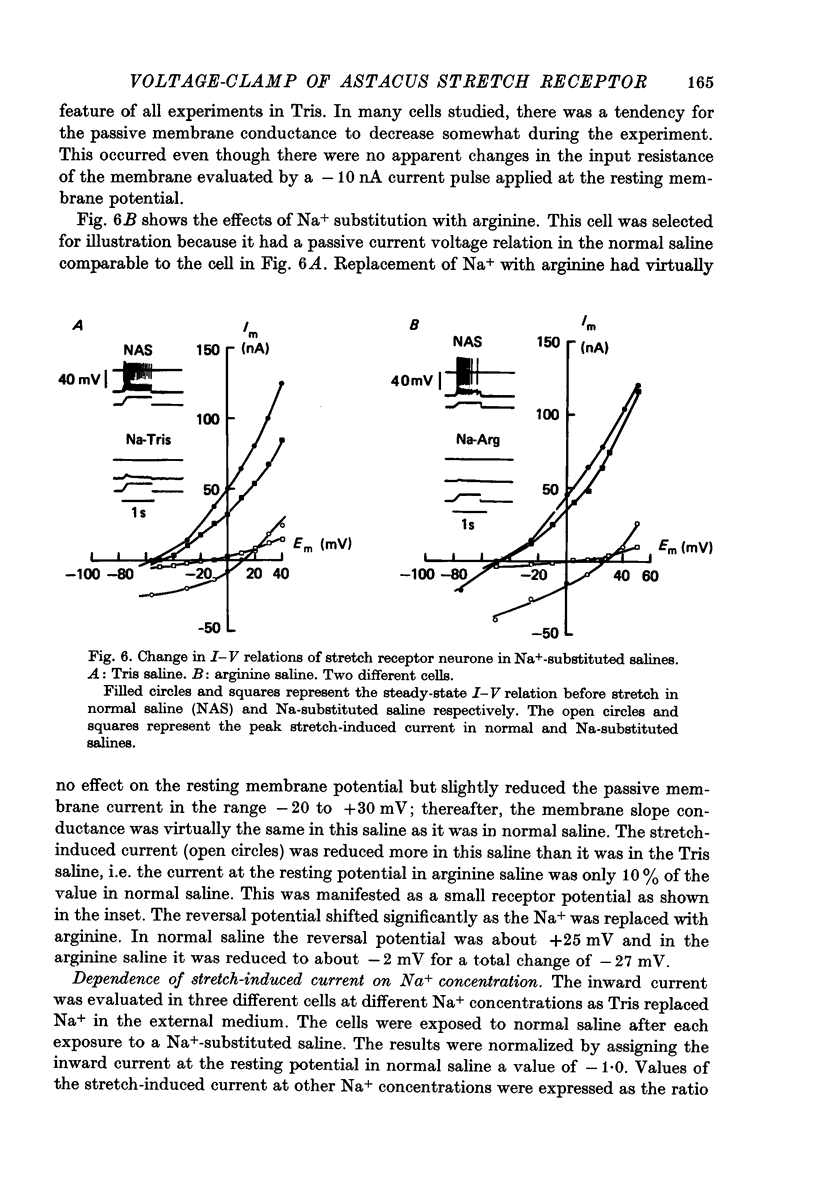
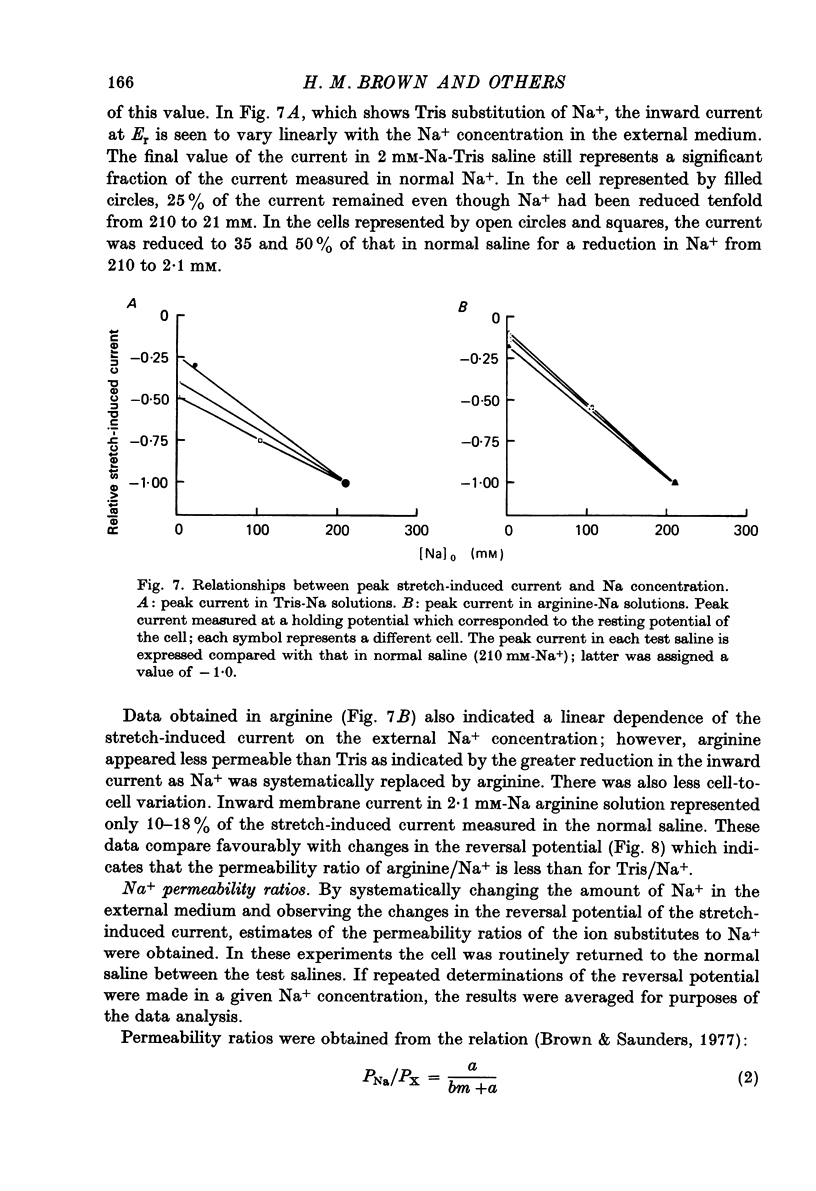
- Klie J. W., Wellhöner H. H. Voltage clamp studies on the stretch response in the neuron of the slowly adapting crayfish stretch receptor. Pflugers Arch. 1973 Aug 17;342(2):93–104. doi: 10.1007/BF00587840. [DOI] [PubMed] [Google Scholar]
- Meyer H., Lux H. D. Action of ammonium on a chloride pump. Removal of hyperpolarizing inhibition in an isolated neuron. Pflugers Arch. 1974;350(2):185–195. doi: 10.1007/BF00586236. [DOI] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. 3. A voltage-clamp study. J Gen Physiol. 1969 Sep;54(3):331–351. doi: 10.1085/jgp.54.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Onodera K. Membrane properties of the stretch receptor neurones of crayfish with particular reference to mechanisms of sensory adaptation. J Physiol. 1969 Jan;200(1):161–185. doi: 10.1113/jphysiol.1969.sp008687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara S. Effects of some organic cations on generator potential of crayfish stretch receptor. J Gen Physiol. 1968 Aug;52(2):363–386. doi: 10.1085/jgp.52.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S., Tsuda K. Membrane permeability change during inhibitory transmitter action in crayfish stretch receptor cell. J Neurophysiol. 1973 Sep;36(5):805–816. doi: 10.1152/jn.1973.36.5.805. [DOI] [PubMed] [Google Scholar]
- Saunders J. H., Brown H. M. Liquid and solid-state Cl- -sensitive microelectrodes. Characteristics and application to intracellular Cl- activity in Balanus photoreceptor. J Gen Physiol. 1977 Oct;70(4):507–530. doi: 10.1085/jgp.70.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERZUOLO C. A., WASHIZU Y. Relation between stimulus strength, generator potential and impulse frequency in stretch receptor of Crustacea. J Neurophysiol. 1962 Jan;25:56–66. doi: 10.1152/jn.1962.25.1.56. [DOI] [PubMed] [Google Scholar]


