Abstract
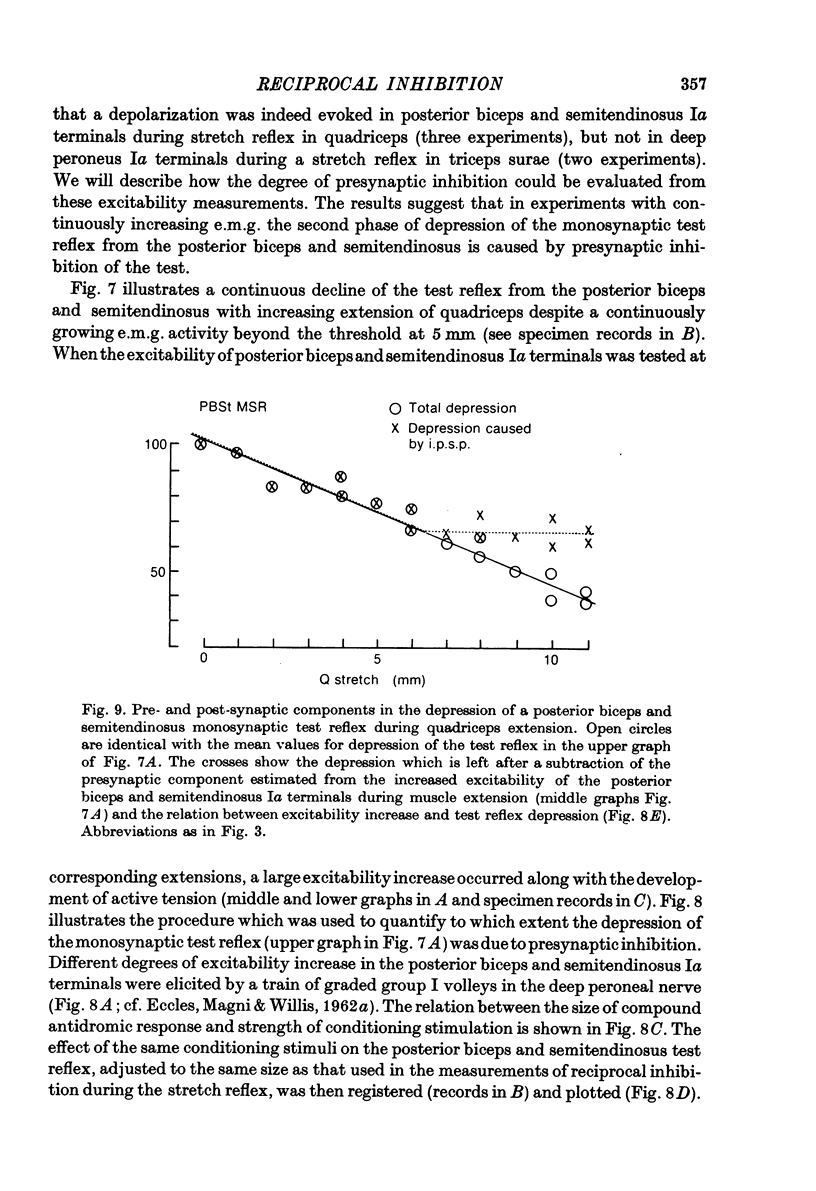
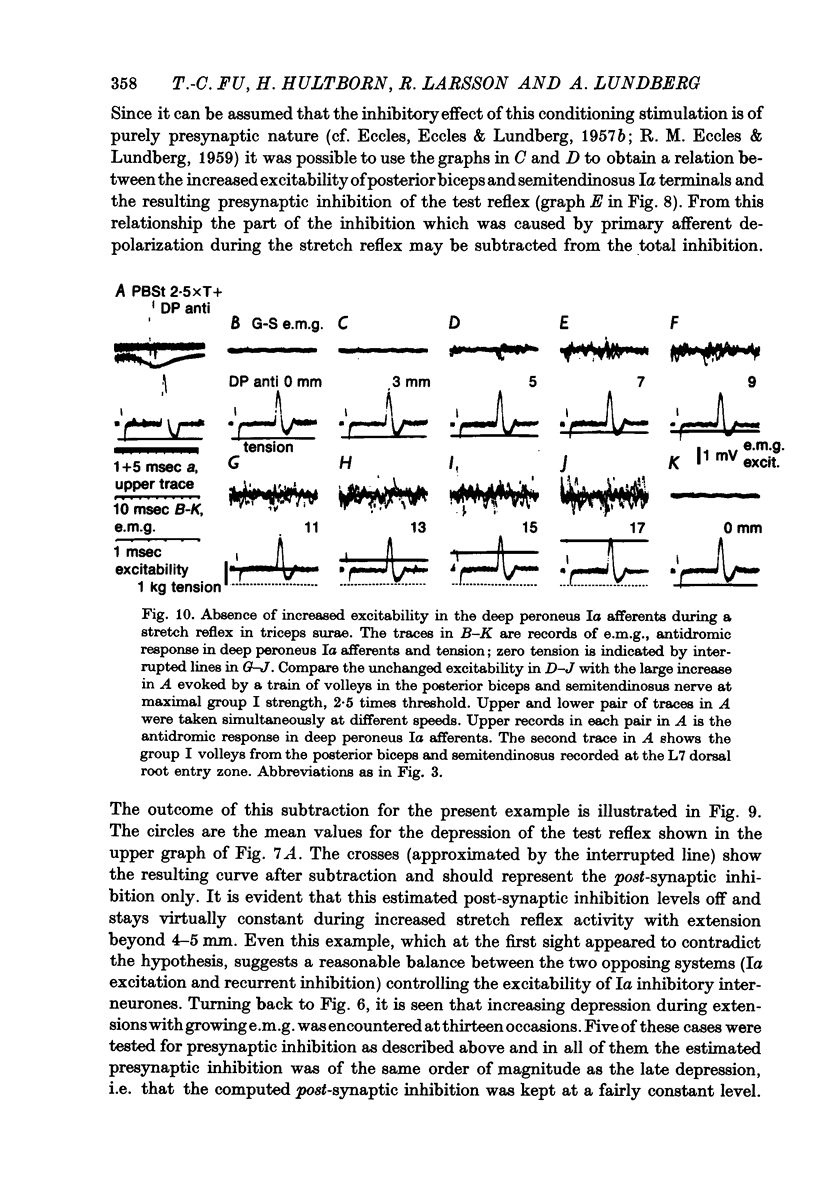
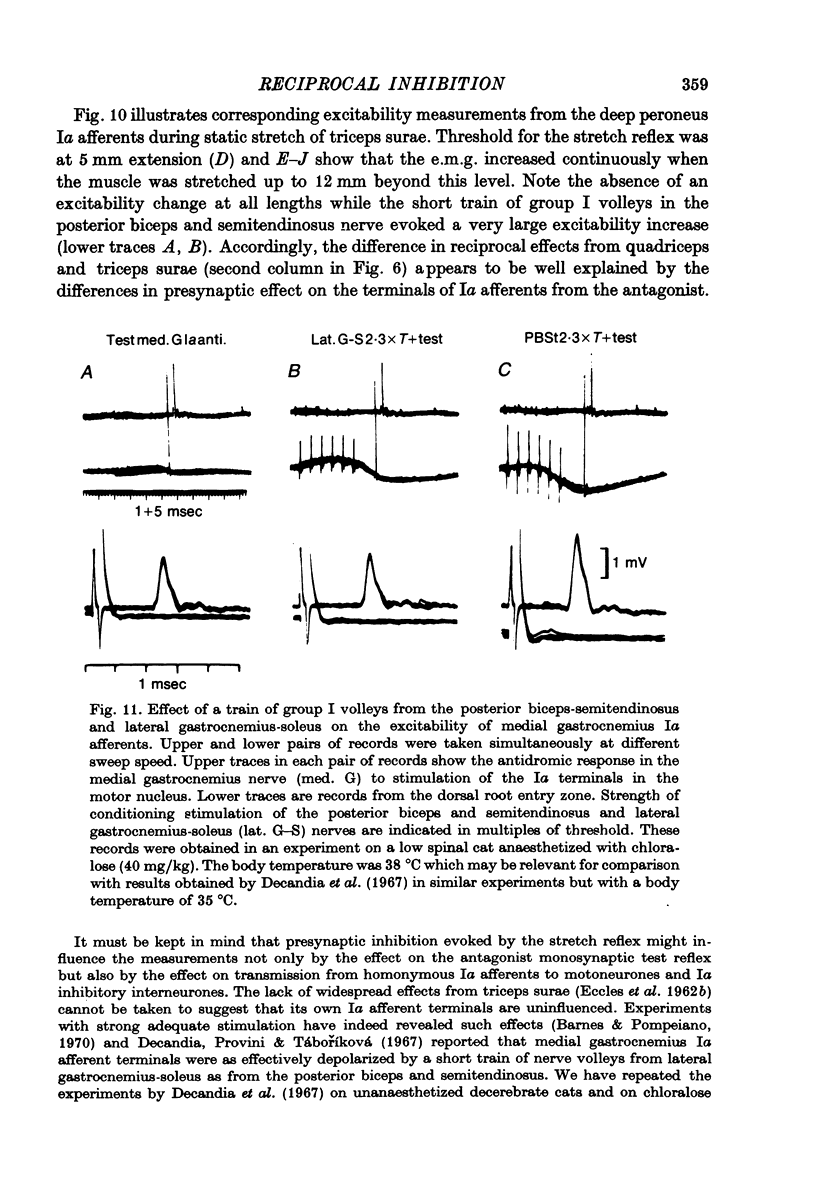
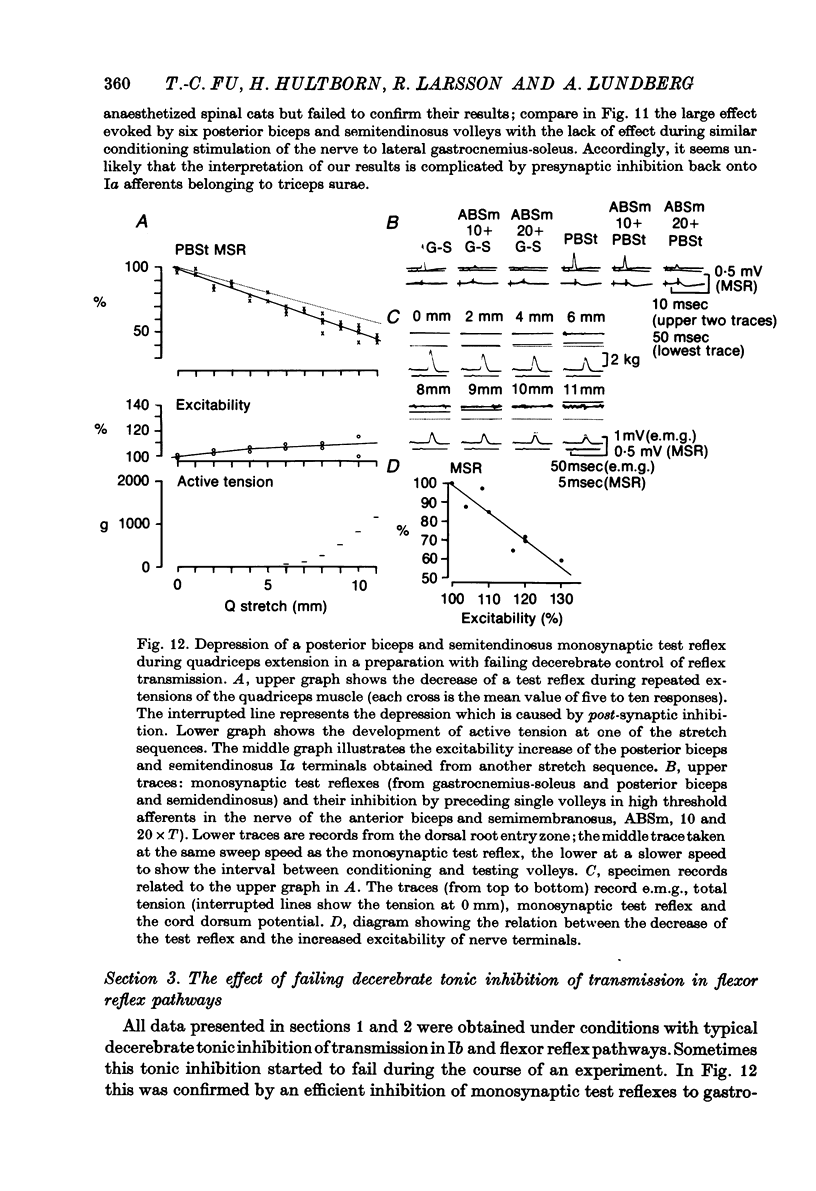
1. The aim of this study was to investigate post-synaptic reciprocal Ia inhibition during the stretch reflex; particularly the extent to which an increased Ia excitation of the Ia inhibitory interneurones will be counteracted by recurrent inhibition from motor axon collaterals. For this purpose we investigated depression of monosynaptic test reflexes antagonist flexors (reciprocal inhibition) during static stretch of quadriceps or triceps surae in unanaesthetized decerebrate cats. 3. With increasing stretch of the extensor muscle there was first a linear augmentation of reciprocal inhibition, but along with the stretch reflex in the extensor a plateau appeared in the inhibition of the flexors, although the extensor stretch reflex (judged by the e.m.g.) increased with further stretching. Within the range of stretching of triceps surae which gave increased stretch reflexes the plateau in the reciprocal inhibition was usually maintained, while during stretching of quadriceps a second phase of augmenting reciprocal inhibition often appeared. Stretch beyond the level which increased the stretch reflex activity gave augmenting reciprocal inhibition both in case of quadriceps and triceps surae. 3. Excitability measurements from central terminals of Ia afferents revealed that the increasing reciprocal inhibition during increasing stretch reflex activity in quadriceps was associated with a primary afferent depolarization in knee flexor Ia afferents; there was no corresponding effect in ankle flexor Ia afferents during stretch reflexes in triceps surae. 4. The primary afferent depolarization evoked in knee flexor Ia afferents by electrical nerve stimulation was then compared with the presynaptic inhibition of knee flexor monosynaptic test reflexes produced by the same stimuli. The results suggest that the second phase of increasing reciprocal inhibition in knee flexors is due to presynaptic inhibition and accordingly that the depth of post-synaptic reciprocal inhibition remains constant at different degrees of stretch reflex activity in both knee and ankle extensors. 5. It is postulated that during increasing stretch reflex activity the increment in Ia excitation and recurrent inhibitio; on to the Ia inhibitory interneurones almost exactly balance each other. It is suggested that recurrent inhibition of Ia inhibitory interneurones may serve as a segmental autoregulatory mechanism to keep 'alpha-gamma-linked reciprocal inhibition' at a constant depth during different levels of agonist activity.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLEY K., EASTON D. M., ECCLES J. C. An investigation of primary or direct inhibition. J Physiol. 1953 Dec 29;122(3):474–488. doi: 10.1113/jphysiol.1953.sp005015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY K., ECCLES J. C. Analysis of the fast afferent impulses from thigh muscles. J Physiol. 1953 Dec 29;122(3):462–473. doi: 10.1113/jphysiol.1953.sp005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C. D., Pompeiano O. Presynaptic and postsynaptic effects in the monosyaptic reflex pathway to extensor motoneurons folowing vibration of synergic muscles. Arch Ital Biol. 1970 Apr;108(2):259–294. [PubMed] [Google Scholar]
- Benecke R., Böttcher U., Henatsch H. D., Meyer-Lohmann J., Schmidt J. Recurrent inhibition of individual Ia inhibitory interneurones and disinhibition of their target alpha-motoneurones during muscle stretches. Exp Brain Res. 1975 Jul 11;23(1):13–28. doi: 10.1007/BF00238726. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Rymer W. Z. Relative strength of synaptic input from short-latency pathways to motor units of defined type in cat medial gastrocnemius. J Neurophysiol. 1976 May;39(3):447–458. doi: 10.1152/jn.1976.39.3.447. [DOI] [PubMed] [Google Scholar]
- CARPENTER D., ENGBERG I., FUNKENSTEIN H., LUNDBERG A. DECEREBRATE CONTROL OF REFLEXES TO PRIMARY AFFERENTS. Acta Physiol Scand. 1963 Dec;59:424–437. doi: 10.1111/j.1748-1716.1963.tb02758.x. [DOI] [PubMed] [Google Scholar]
- Clamann H. P., Henneman E. Electrical measurement of axon diameter and its use in relating motoneuron size to critical firing level. J Neurophysiol. 1976 Jul;39(4):844–851. doi: 10.1152/jn.1976.39.4.844. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., IGGO A., ITO M. Distribution of recurrent inhibition among motoneurones. J Physiol. 1961 Dec;159:479–499. doi: 10.1113/jphysiol.1961.sp006822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J Physiol. 1957 Sep 30;138(2):227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones in relation to the two components of the group I muscle afferent volley. J Physiol. 1957 May 23;136(3):527–546. doi: 10.1113/jphysiol.1957.sp005778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R. F., WILLIS W. D. Presynaptic inhibition of the spinal monosynaptic reflex pathway. J Physiol. 1962 May;161:282–297. doi: 10.1113/jphysiol.1962.sp006886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Supraspinal control of interneurones mediating spinal reflexes. J Physiol. 1959 Oct;147:565–584. doi: 10.1113/jphysiol.1959.sp006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELDRED E., GRANIT R., MERTON P. A. Supraspinal control of the muscle spindles and its significance. J Physiol. 1953 Dec 29;122(3):498–523. doi: 10.1113/jphysiol.1953.sp005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Magni F., Willis W. D. Depolarization of central terminals of Group I afferent fibres from muscle. J Physiol. 1962 Jan;160(1):62–93. doi: 10.1113/jphysiol.1962.sp006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedina L., Hultborn H. Facilitation from ipsilateral primary afferents of interneuronal transmission in the Ia inhibitory pathway to motoneurones. Acta Physiol Scand. 1972 Sep;86(1):59–81. doi: 10.1111/j.1748-1716.1972.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Fedina L., Hultborn H., Illert M. Facilitation from contralateral primary afferents of interneuronal transmission in the Ia inhibitory pathway to motoneurones. Acta Physiol Scand. 1975 Jun;94(2):198–221. doi: 10.1111/j.1748-1716.1975.tb05880.x. [DOI] [PubMed] [Google Scholar]
- GRANIT R. Neuromuscular interaction in postural tone of the cat's isometric soleus muscle. J Physiol. 1958 Oct 31;143(3):387–402. doi: 10.1113/jphysiol.1958.sp006067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., PASCOE J. E., STEG G. The behaviour of tonic alpha and gamma motoneurones during stimulation of recurrent collaterals. J Physiol. 1957 Oct 30;138(3):381–400. doi: 10.1113/jphysiol.1957.sp005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. The role of muscle stiffness in meeting the changing postural and locomotor requirements for force development by the ankle extensors. Acta Physiol Scand. 1972 Sep;86(1):92–108. doi: 10.1111/j.1748-1716.1972.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Grillner S., Udo M. Motor unit activity and stiffness of the contracting muscle fibres in the tonic stretch reflex. Acta Physiol Scand. 1971 Mar;81(3):422–424. doi: 10.1111/j.1748-1716.1971.tb04916.x. [DOI] [PubMed] [Google Scholar]
- Grillner S., Udo M. Recruitment in the tonic stretch reflex. Acta Physiol Scand. 1971 Apr;81(4):571–573. doi: 10.1111/j.1748-1716.1971.tb04935.x. [DOI] [PubMed] [Google Scholar]
- Gydikov A., Kosarov D. Some features of different motor units in human biceps brachii. Pflugers Arch. 1974 Feb 18;347(1):75–88. doi: 10.1007/BF00587056. [DOI] [PubMed] [Google Scholar]
- HARVEY R. J., MATTHEWS P. B. The response of de-efferented muscle spindle endings in the cat's soleus to slow extension of the muscle. J Physiol. 1961 Jul;157:370–392. doi: 10.1113/jphysiol.1961.sp006729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. II. Facilitation of interneuronal transmission in reflex paths to motoneurones. Exp Brain Res. 1969;7(4):365–391. doi: 10.1007/BF00237321. [DOI] [PubMed] [Google Scholar]
- Houk J. C., Singer J. J., Goldman M. R. An evaluation of length and force feedback to soleus muscles of decerebrate cats. J Neurophysiol. 1970 Nov;33(6):784–811. doi: 10.1152/jn.1970.33.6.784. [DOI] [PubMed] [Google Scholar]
- Hultborn H. Convergence on interneurones in the reciprocal Ia inhibitory pathway to motoneurones. Acta Physiol Scand Suppl. 1972;375:1–42. doi: 10.1111/j.1748-1716.1972.tb05298.x. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Illert M., Santini M. Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones. I. Disynaptic Ia inhibition of Ia inhibitory interneurones. Acta Physiol Scand. 1976 Feb;96(2):193–201. doi: 10.1111/j.1748-1716.1976.tb10188.x. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Illert M., Santini M. Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones. II. Effects from segmental flexor reflex pathways. Acta Physiol Scand. 1976 Mar;96(3):351–367. doi: 10.1111/j.1748-1716.1976.tb10205.x. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Illert M., Santini M. Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones. III. Effects from supraspinal pathways. Acta Physiol Scand. 1976 Mar;96(3):368–391. doi: 10.1111/j.1748-1716.1976.tb10206.x. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurones. J Physiol. 1971 Jul;215(3):591–612. doi: 10.1113/jphysiol.1971.sp009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition of interneurones monosynaptically activated from group Ia afferents. J Physiol. 1971 Jul;215(3):613–636. doi: 10.1113/jphysiol.1971.sp009488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Relative contribution from different nerves to recurrent depression of Ia IPSPs in motoneurones. J Physiol. 1971 Jul;215(3):637–664. doi: 10.1113/jphysiol.1971.sp009489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S., Roberts W. Neuronal pathway of the recurrent facilitation of motoneurones. J Physiol. 1971 Oct;218(2):495–514. doi: 10.1113/jphysiol.1971.sp009630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Lundberg A. Reciprocal inhibition during the stretch reflex. Acta Physiol Scand. 1972 May;85(1):136–138. doi: 10.1111/j.1748-1716.1972.tb05243.x. [DOI] [PubMed] [Google Scholar]
- Hultborn H. Transmission in the pathway of reciprocal Ia inhibition to motoneurones and its control during the tonic stretch reflex. Prog Brain Res. 1976;44:235–255. doi: 10.1016/S0079-6123(08)60736-0. [DOI] [PubMed] [Google Scholar]
- JANSEN J. K., MATTHEWS P. B. The effects of fusimotor activity on the static responsiveness of primary and secondary endings of muscle spindles in the decerebrate cat. Acta Physiol Scand. 1962 Aug;55:376–386. doi: 10.1111/j.1748-1716.1962.tb02451.x. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Roberts R. C. The role of muscle spindle afferents in stretch and vibration reflexes of the soleus muscle of the decerebrate cat. Brain Res. 1978 May 12;146(2):366–372. doi: 10.1016/0006-8993(78)90981-2. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Monosynaptic excitation of motoneurones from muscle spindle secondary endings of intercostal and triceps surae muscles in the cat. J Physiol. 1975 Feb;245(2):64P–66P. [PubMed] [Google Scholar]
- Lund S., Lundberg A., Vyklický L. Inhibitory action from the flexor reflex afferents on transmission to Ia afferents. Acta Physiol Scand. 1965 Aug;64(4):345–355. doi: 10.1111/j.1748-1716.1965.tb04189.x. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Comments on reflex actions evoked by electrical stimulation of group II muscle afferents. Brain Res. 1977 Feb 25;122(3):551–555. doi: 10.1016/0006-8993(77)90466-8. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. THE RESPONSE OF DE-EFFERENTED MUSCLE SPINDLE RECEPTORS TO STRETCHING AT DIFFERENT VELOCITIES. J Physiol. 1963 Oct;168:660–678. doi: 10.1113/jphysiol.1963.sp007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryall R. W., Piercey M. F., Polosa C., Goldfarb J. Excitation of Renshaw cells in relation to orthodromic and antidromic excitation of motoneurons. J Neurophysiol. 1972 Jan;35(1):137–148. doi: 10.1152/jn.1972.35.1.137. [DOI] [PubMed] [Google Scholar]
- Stauffer E. K., Stephens J. A. The tendon organs of cat soleus: static sensitivity to active force. Exp Brain Res. 1975 Sep 29;23(3):279–291. doi: 10.1007/BF00239740. [DOI] [PubMed] [Google Scholar]
- Stauffer E. K., Watt D. G., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 2. Spindle group II afferents. J Neurophysiol. 1976 Nov;39(6):1393–1402. doi: 10.1152/jn.1976.39.6.1393. [DOI] [PubMed] [Google Scholar]


