Abstract
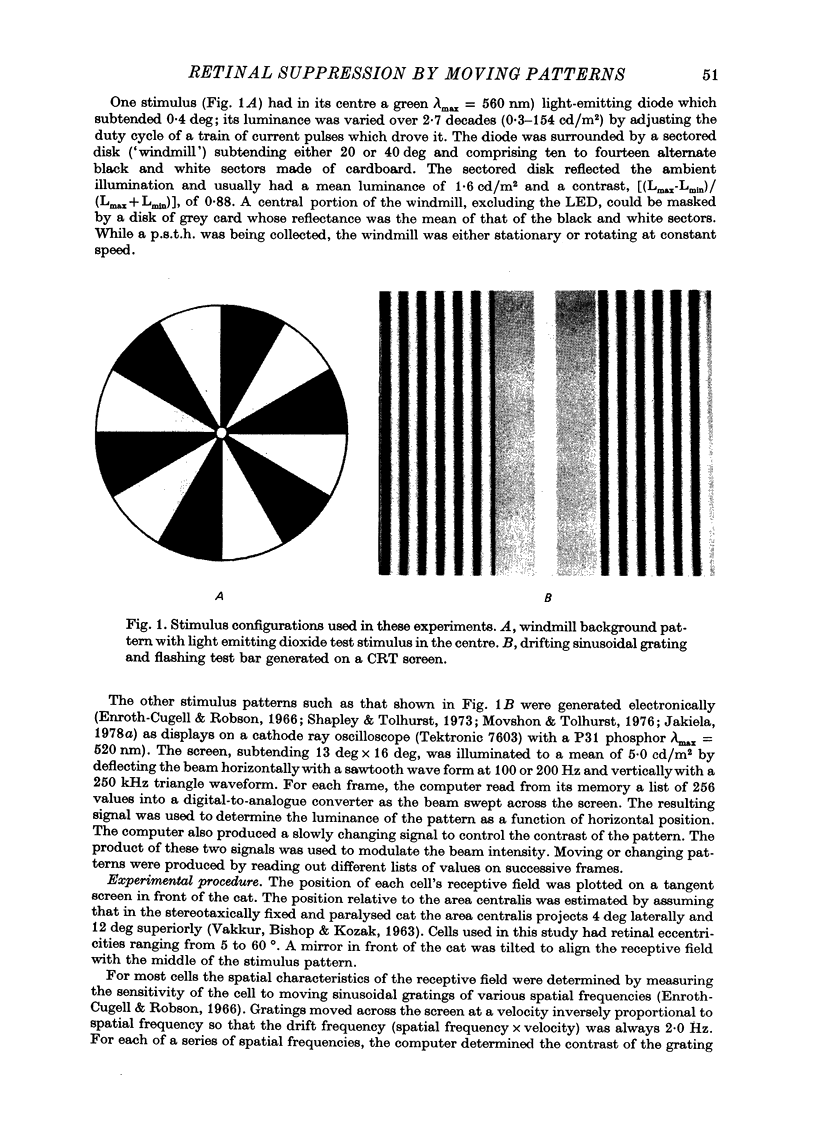
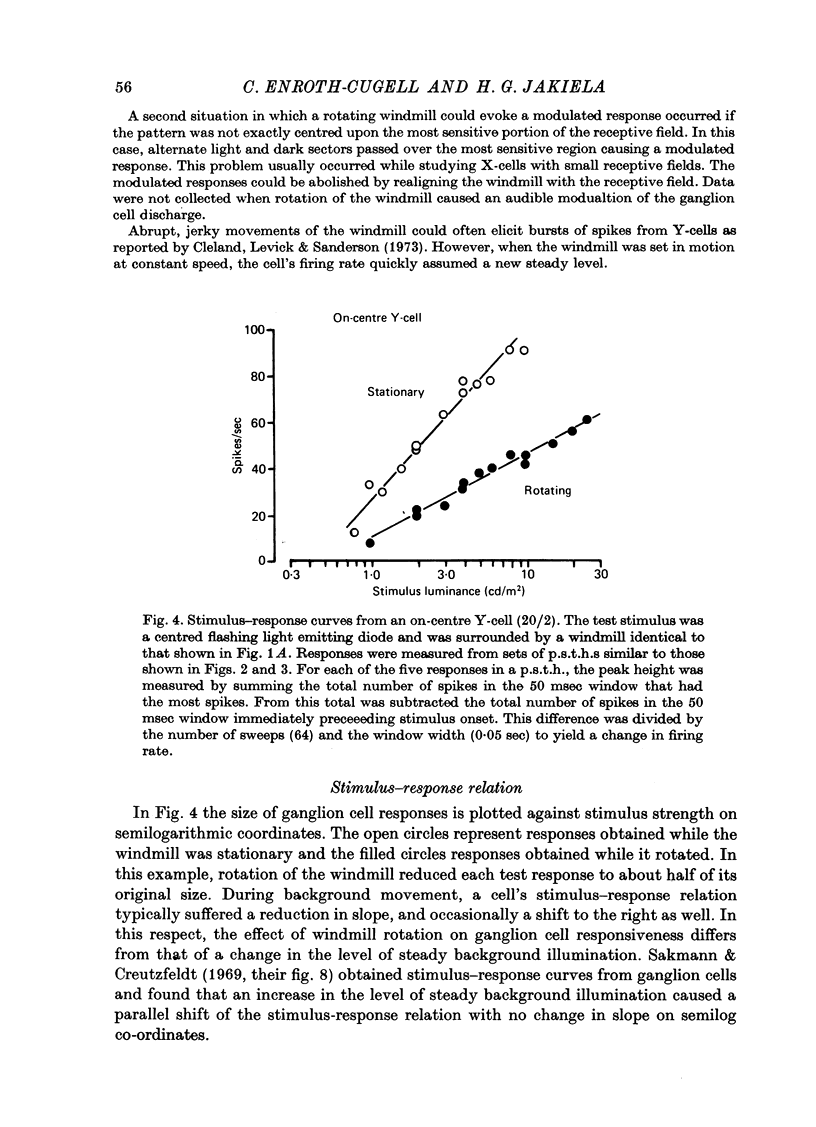
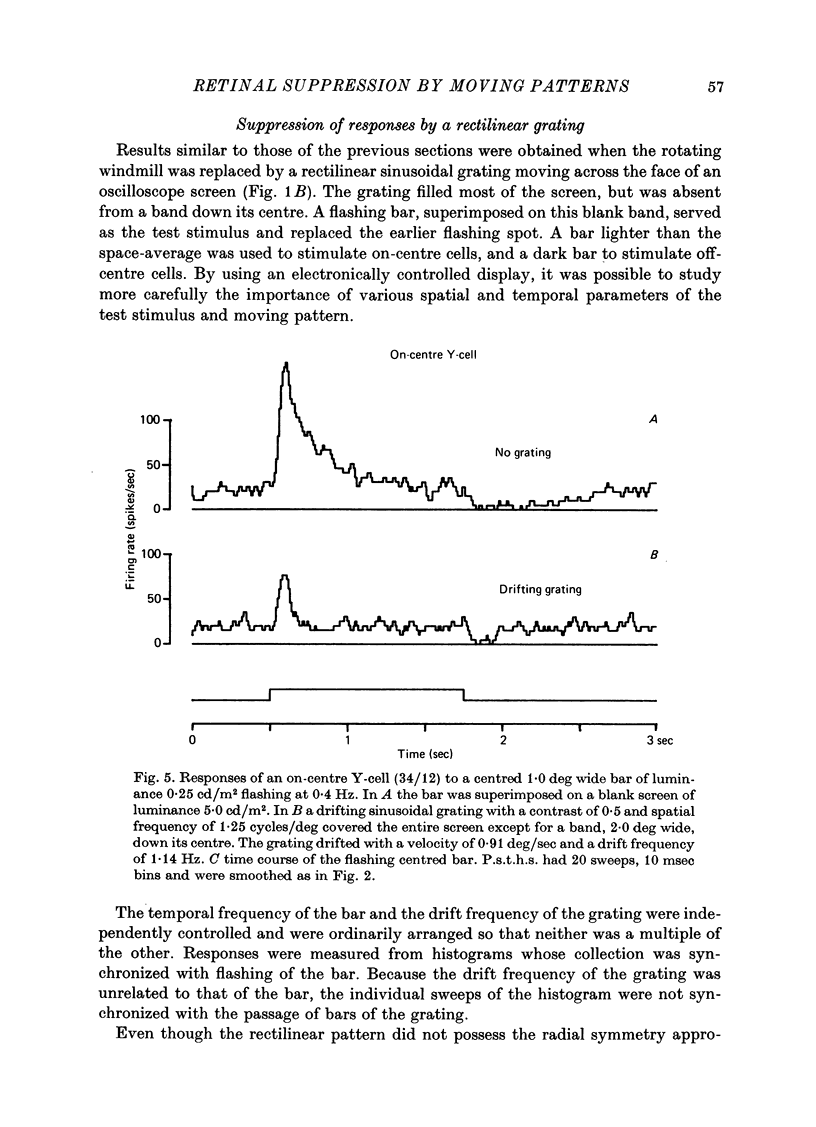
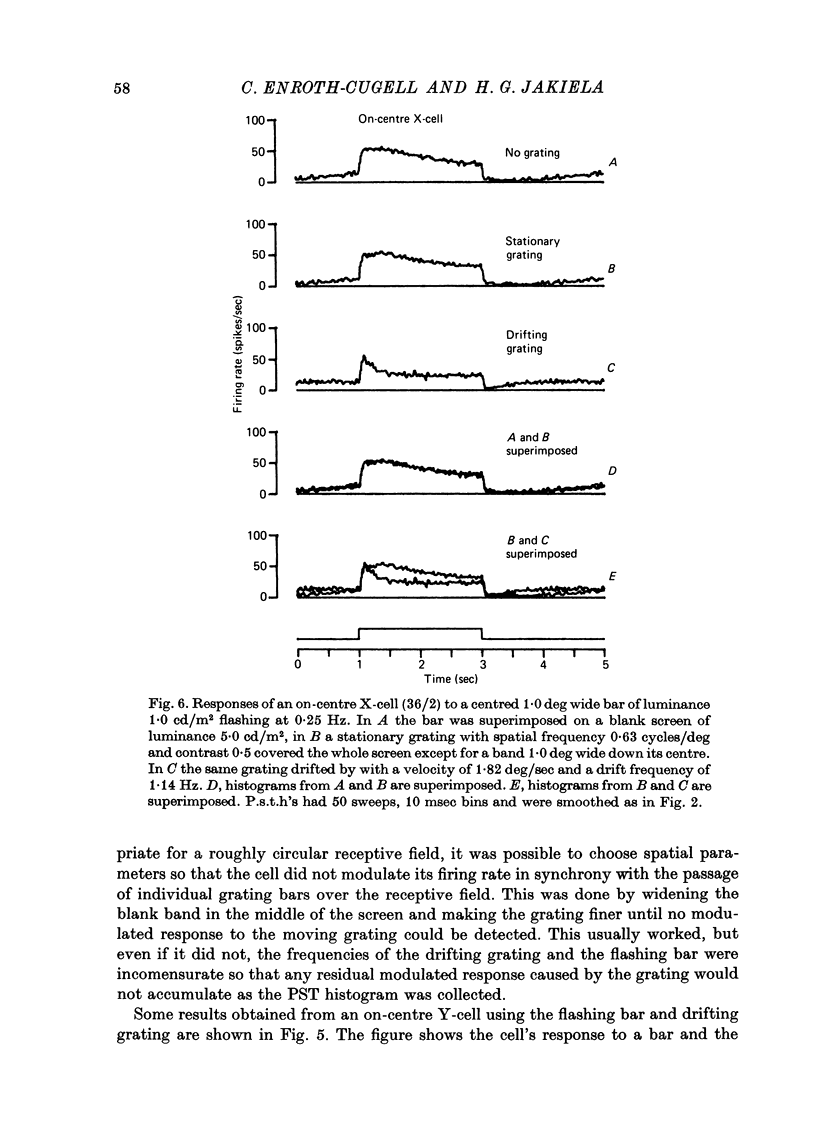
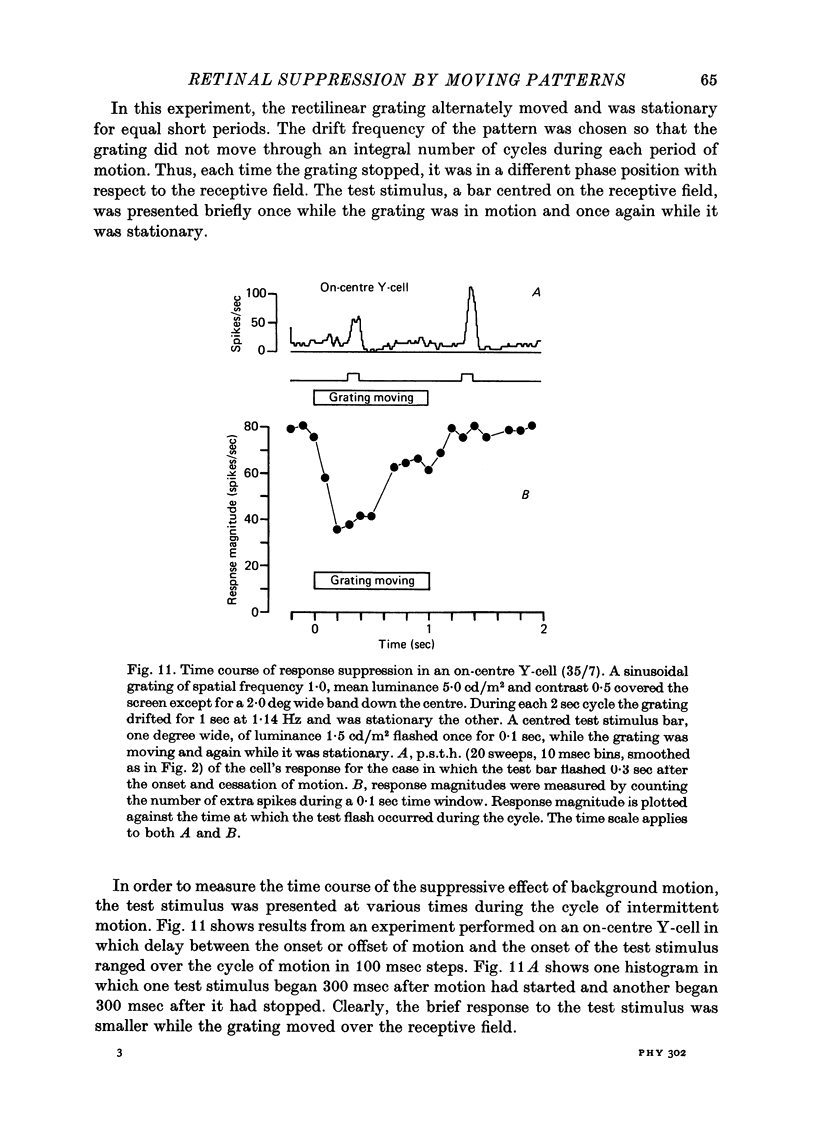
1. Action potentials were recorded from single fibres in the optic tract of anaesthetized cats. 2. A sectored disk or 'windmill', concentric with the receptive field, was rotated about its centre to cause local changes in illumination throughout the receptive field without changing the total amount of light falling on the receptive field centre or surround. 3. A cell's response to a flashing test spot centered on its receptive field was measured both while the windmill was stationary and while it rotated. While the windmill rotated, the test spot evoked a smaller average number of spikes than while the windmill was stationary. 4. The induction in response occurred in both on-centre and off-centre cells and in both X-cells and Y-cells, though the reduction in response was smaller in X-cells. 5. Surround responses, evoked by an eccentric stimulus, were also reduced by a moving peripheral pattern. 6. Suppression was graded with the contrast of the moving pattern. 7. Gratings too fine to be resolved by the receptive field centre could suppress the response of Y-cells. This suggests that the local elements responsible for the suppression are smaller than the receptive field centres of Y-cells. 8. Response suppression started within the 100 msec of the onset of pattern motion.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B., Derrington A. M., Harris L. R., Lennie P. The effects of remote retinal stimulation on the responses of cat retinal ganglion cells. J Physiol. 1977 Jul;269(1):177–194. doi: 10.1113/jphysiol.1977.sp011898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Three factors limiting the reliable detection of light by retinal ganglion cells of the cat. J Physiol. 1969 Jan;200(1):1–24. doi: 10.1113/jphysiol.1969.sp008679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-Cugell C. Quantitative aspects of gain and latency in the cat retina. J Physiol. 1970 Jan;206(1):73–91. doi: 10.1113/jphysiol.1970.sp008998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Properties of rarely encountered types of ganglion cells in the cat's retina and an overall classification. J Physiol. 1974 Jul;240(2):457–492. doi: 10.1113/jphysiol.1974.sp010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Sanderson K. J. Properties of sustained and transient ganglion cells in the cat retina. J Physiol. 1973 Feb;228(3):649–680. doi: 10.1113/jphysiol.1973.sp010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch J. M., Lazarus J., Johnson C. A. Human psychophysical analysis of receptive field-like properties. I. A new transient-like visual response using a moving windmill (Werblin-type) target. Sens Processes. 1976 Jun;1(1):14–32. [PubMed] [Google Scholar]
- Enroth-Cugell C., Lennie P. The control of retinal ganglion cell discharge by receptive field surrounds. J Physiol. 1975 Jun;247(3):551–578. doi: 10.1113/jphysiol.1975.sp010947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. H. Properties of the surround response mechanism of cat retinal ganglion cells and centre-surround interaction. J Physiol. 1972 Jan;220(2):403–439. doi: 10.1113/jphysiol.1972.sp009714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. Algebraic summation of centre and surround inputs to retinal ganglion cells of the cat. Nature. 1970 May 2;226(5244):458–459. doi: 10.1038/226458a0. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Adaptation and dynamics of cat retinal ganglion cells. J Physiol. 1973 Sep;233(2):271–309. doi: 10.1113/jphysiol.1973.sp010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Flux, not retinal illumination, is what cat retinal ganglion cells really care about. J Physiol. 1973 Sep;233(2):311–326. doi: 10.1113/jphysiol.1973.sp010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B., Krüger J., Droll W. Quantitative aspects of the shift-effect in cat retinal ganglion cells. Brain Res. 1975 Jan 17;83(3):391–403. doi: 10.1016/0006-8993(75)90832-x. [DOI] [PubMed] [Google Scholar]
- Hickey T. L., Winters R. W., Pollack J. G. Center-surround interactions in two types of on-center retinal ganglion cells in the cat. Vision Res. 1973 Aug;13(8):1511–1526. doi: 10.1016/0042-6989(73)90010-2. [DOI] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. A., Enoch J. M. Human psychophysical analysis of receptive field-like properties--III. Dichoptic properties of a new transient-like psychophysical function. Vision Res. 1976;16(12):1463–1470. doi: 10.1016/0042-6989(76)90166-8. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A. Receptive field organization of bipolar and amacrine cells in the goldfish retina. J Physiol. 1973 Nov;235(1):133–153. doi: 10.1113/jphysiol.1973.sp010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick W. R., Cleland B. G., Dubin M. W. Lateral geniculate neurons of cat: retinal inputs and physiology. Invest Ophthalmol. 1972 May;11(5):302–311. [PubMed] [Google Scholar]
- MCILWAIN J. T. RECEPTIVE FIELDS OF OPTIC TRACT AXONS AND LATERAL GENICULATE CELLS: PERIPHERAL EXTENT AND BARBITURATE SENSITIVITY. J Neurophysiol. 1964 Nov;27:1154–1173. doi: 10.1152/jn.1964.27.6.1154. [DOI] [PubMed] [Google Scholar]
- Mackay D. M. Elevation of visual threshold by displacement of retinal image. Nature. 1970 Jan 3;225(5227):90–92. doi: 10.1038/225090a0. [DOI] [PubMed] [Google Scholar]
- Mateeff S., Yakimoff N., Mitrani L. Some characteristics of the visual masking by moving contours. Vision Res. 1976;16(5):489–492. doi: 10.1016/0042-6989(76)90027-4. [DOI] [PubMed] [Google Scholar]
- Matin E. Saccadic suppression: a review and an analysis. Psychol Bull. 1974 Dec;81(12):899–917. doi: 10.1037/h0037368. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Tolhurst D. J. Proceedings: The use of a digital computer in the study of neuronal properties in the visual system. J Physiol. 1976 Jan;254(1):2P–4P. [PubMed] [Google Scholar]
- RUSHTON W. A. VISUAL ADAPTATION. Proc R Soc Lond B Biol Sci. 1965 Mar 16;162:20–46. doi: 10.1098/rspb.1965.0024. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W. Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vision Res. 1965 Dec;5(11):583–601. doi: 10.1016/0042-6989(65)90033-7. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Response of cat retinal ganglion cells to moving visual patterns. J Neurophysiol. 1965 Sep;28(5):819–832. doi: 10.1152/jn.1965.28.5.819. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Creutzfeldt O. D. Scotopic and mesopic light adaptation in the cat's retina. Pflugers Arch. 1969;313(2):168–185. doi: 10.1007/BF00586245. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Organization of on-off cells in the retina of the turtle. J Physiol. 1973 Apr;230(1):1–14. doi: 10.1113/jphysiol.1973.sp010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R. M., Tolhurst D. J. Edge detectors in human vision. J Physiol. 1973 Feb;229(1):165–183. doi: 10.1113/jphysiol.1973.sp010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R. M., Victor J. D. Nonlinear spatial summation and the contrast gain control of cat retinal ganglion cells. J Physiol. 1979 May;290(2):141–161. doi: 10.1113/jphysiol.1979.sp012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R. M., Victor J. D. The effect of contrast on the transfer properties of cat retinal ganglion cells. J Physiol. 1978 Dec;285:275–298. doi: 10.1113/jphysiol.1978.sp012571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibos L. N., Werblin F. S. The properties of surround antagonism elicited by spinning windmill patterns in the mudpuppy retina. J Physiol. 1978 May;278:101–116. doi: 10.1113/jphysiol.1978.sp012295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda J., Hashimoto H., Otsu K. Bipolar-amacrine transmission in the carp retina. Vision Res. 1973 Feb;13(2):295–307. doi: 10.1016/0042-6989(73)90108-9. [DOI] [PubMed] [Google Scholar]
- VAKKUR G. J., BISHOP P. O., KOZAK W. VISUAL OPTICS IN THE CAT, INCLUDING POSTERIOR NODAL DISTANCE AND RETINAL LANDMARKS. Vision Res. 1963 Nov;61:289–314. doi: 10.1016/0042-6989(63)90004-x. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Copenhagen D. R. Control of retinal sensitivity. 3. Lateral interactions at the inner plexiform layer. J Gen Physiol. 1974 Jan;63(1):88–110. doi: 10.1085/jgp.63.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. The control of sensitivity in the retina. Sci Am. 1973 Jan;228(1):70–79. doi: 10.1038/scientificamerican0173-70. [DOI] [PubMed] [Google Scholar]



