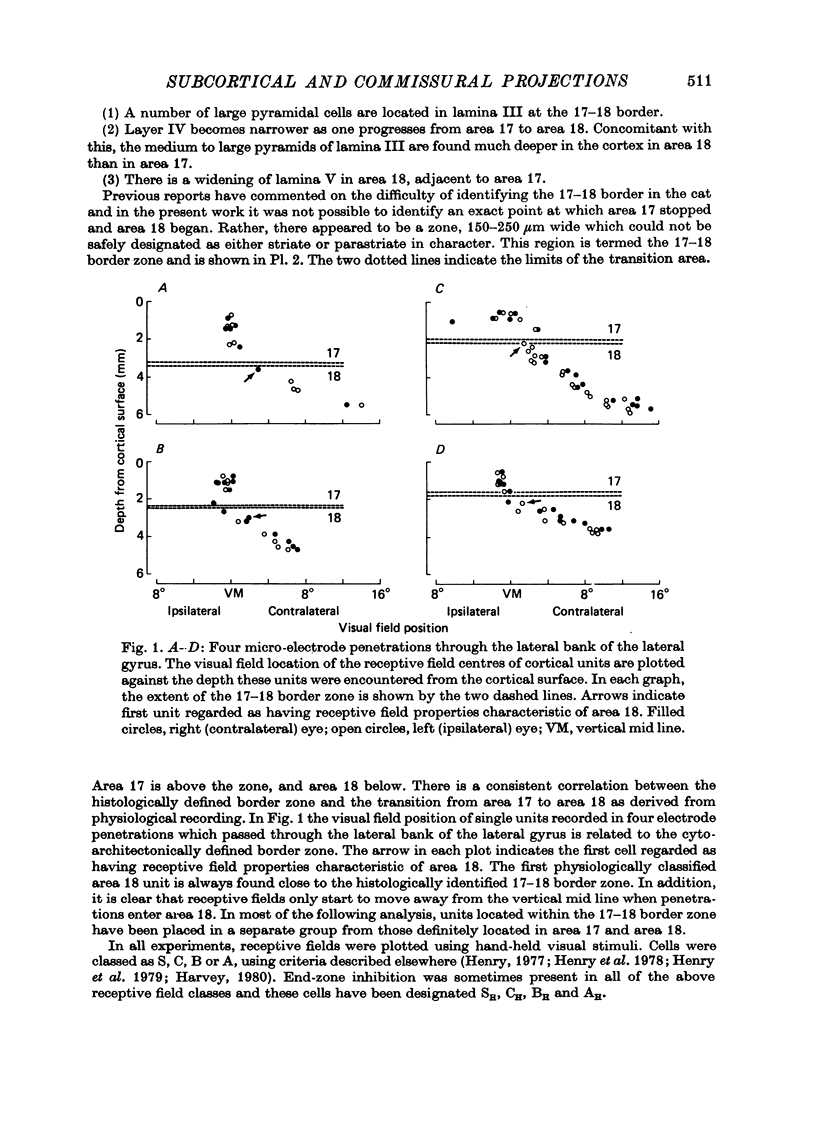
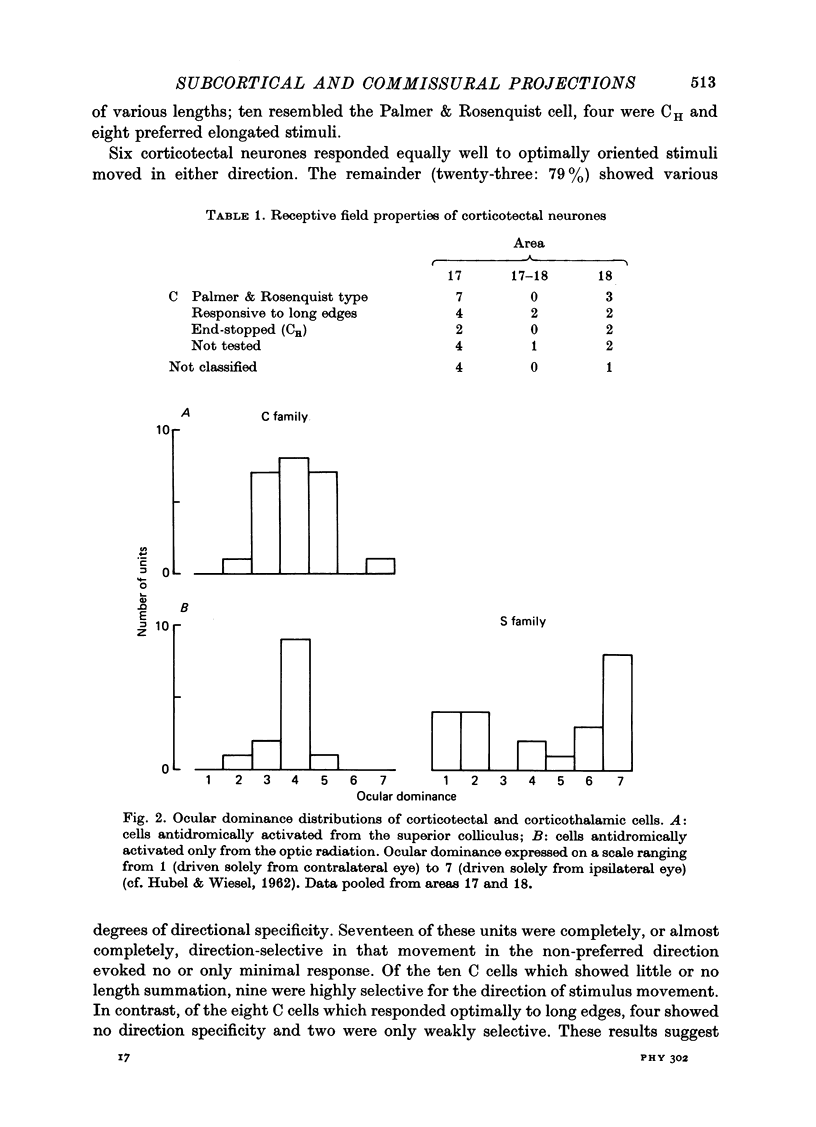
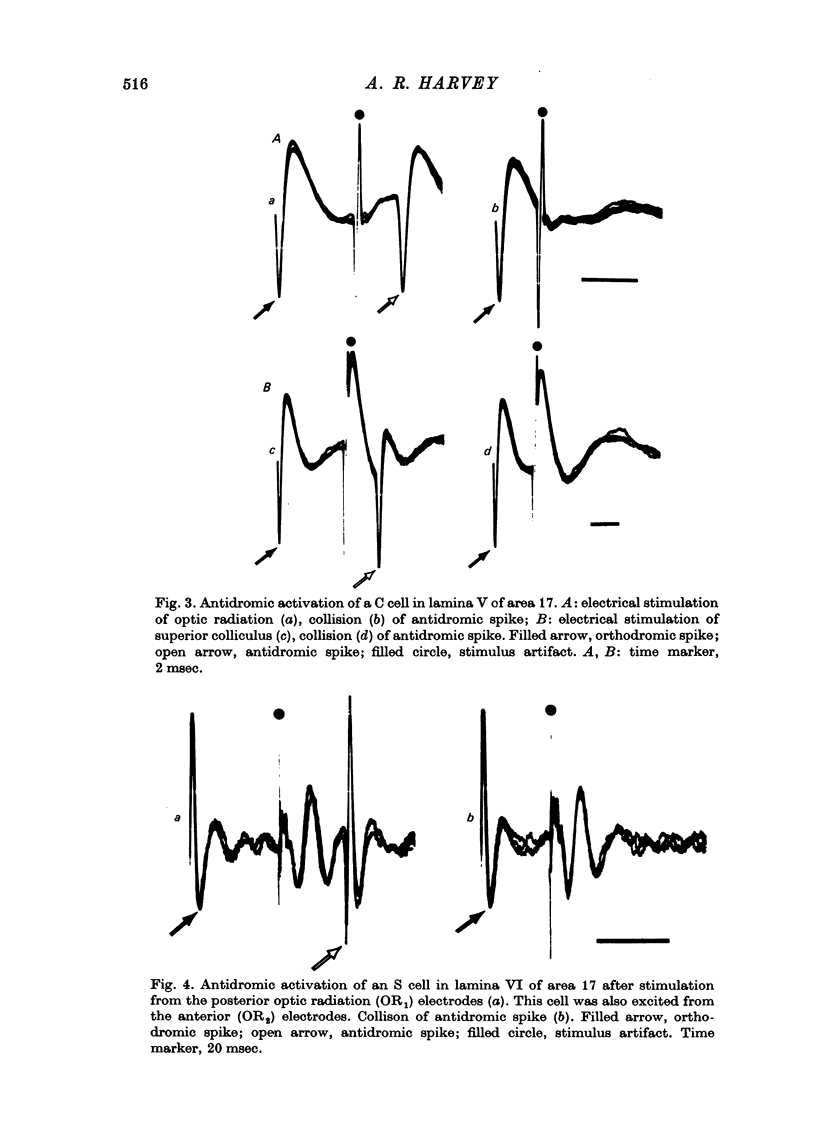
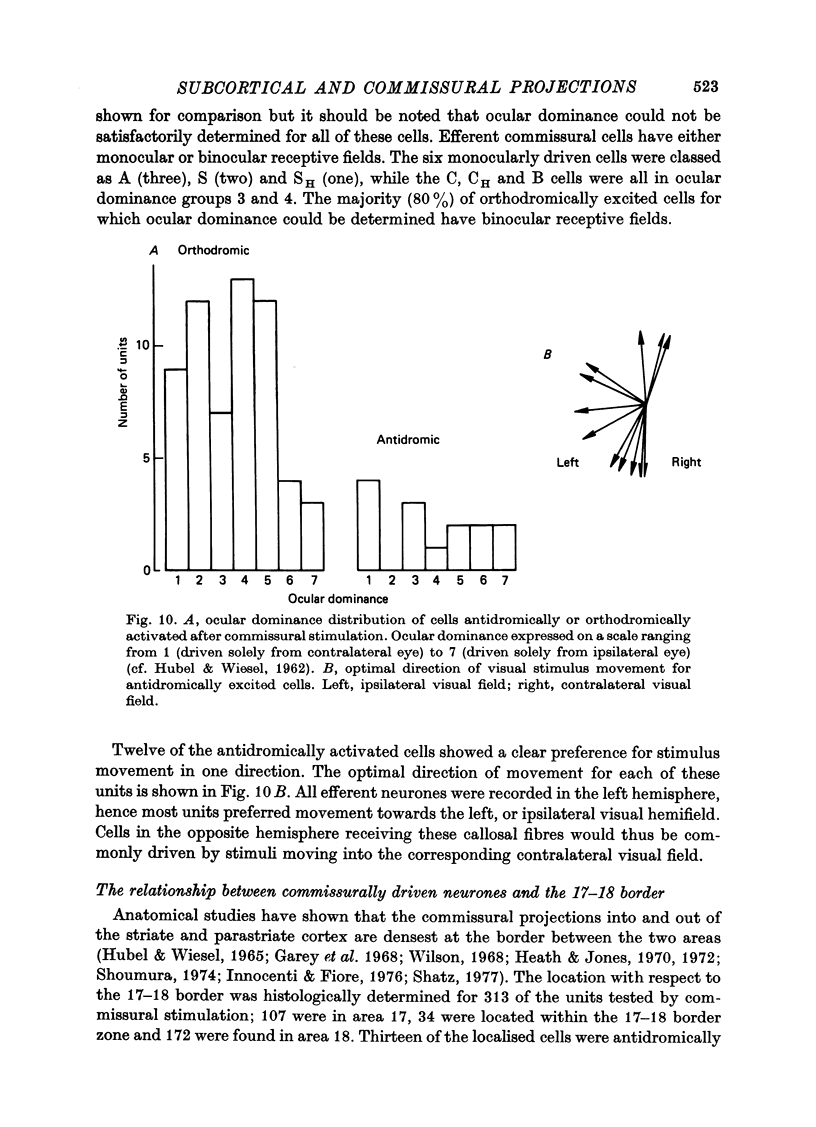
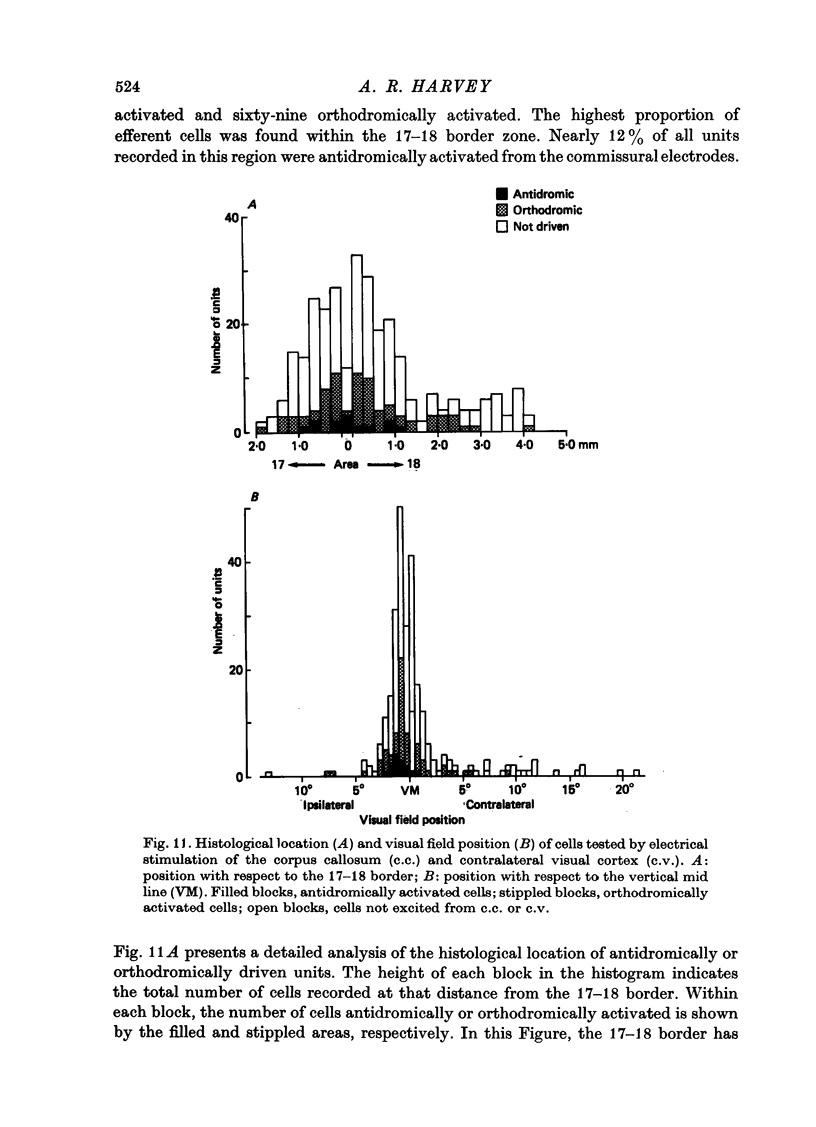
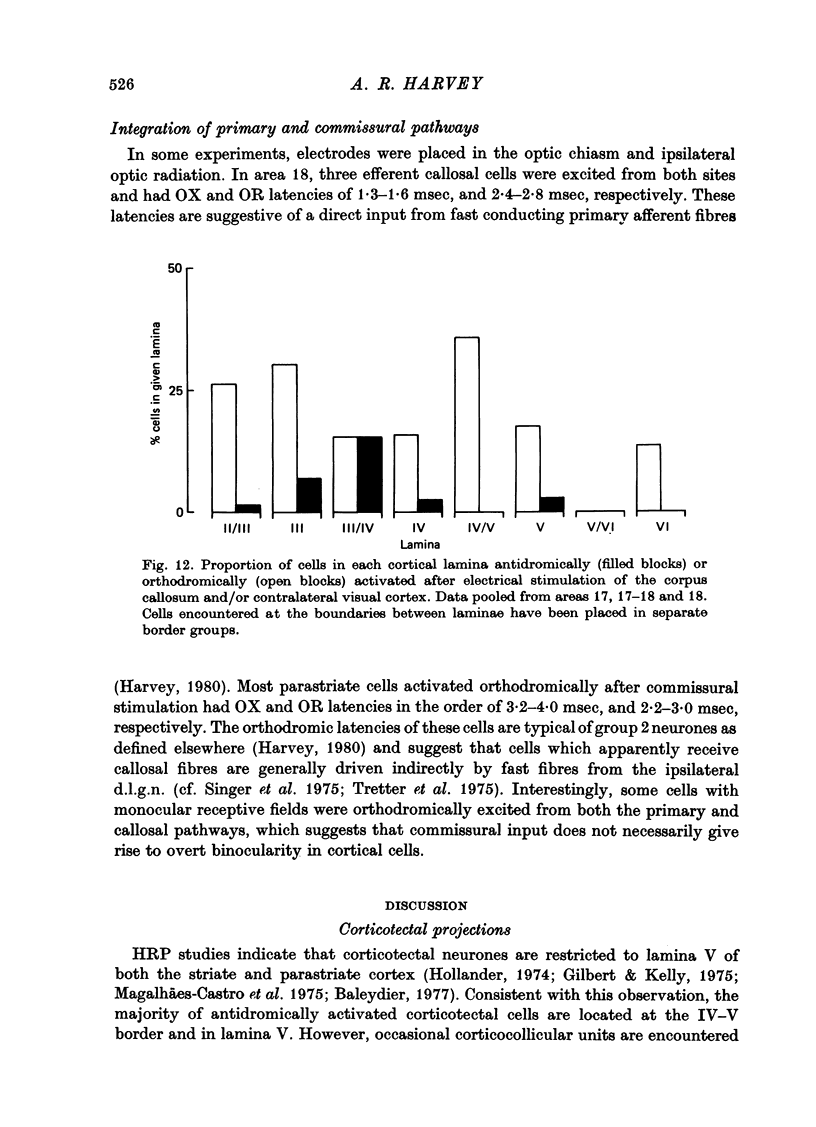
Abstract
1. The corticotectal, corticothalamic and commissural projections of areas 17 and 18 of the cat have been examined using electrical stimulation techniques. 2. In both area 17 and area 18, almost all corticotectal neurones are C cells and have binocular receptive fields. Some of these cells respond equally well to both small moving spots and elongated stimuli, while others only respond to stimuli of restricted length (cf. Palmer & Rosenquist, 1974). Both types are highly direction-selective. A third type of corticotectal C cell responds optimally to long edges or bars and shows only weak direction selectivity. Corticotectal cells generally have fast conducting axons and the majority are encountered in lamina V. About 25% of all cells recorded in lamina V can be antidromically activated from the superior colliculus. 3. Striate and parastriate cells efferent to the thalamus can have either S or C type receptive fields. Corticothalamic S cells are the most common type of efferent cell in lamina VI and have more slowly conducting axons than C cells. Efferent S cells are almost always direction-selective and about half have binocular receptive fields. 4. It is suggested that there may be at least three subgroups within the corticothalamic cells: lamina V C cells project to the pulvinare complex (the same cells may also send axons to the superior colliculus), lamina VI C cells project to the perigeniculate nucleus and lamina VI S cells provide the cortical input to neurones within the lateral geniculate nucleus. 5. In contrast to the corticotectal and corticothalamic projections, the receptive fields of cells projecting through the corpus callosum forth a heterogenous group. All major striate and parastriate receptive field classes are efferent to the contralateral cortex. Their receptive field centres are located close to the vertical mid line and most cells respond best to stimuli moving towards the ipsilateral visual hemifield. Efferent neurones are mostly encountered in lamina III, within about 1mm either side of the 17-18 border zone. 6. Cells orthodromically excited after commissural stimulation have mostly C or B type receptive fields. Unlike efferent callosal neurones, orthodromically activated cells are encountered up to 3 mm into area 18 and can have receptive fields located up to 9 degrees from the vertical mid line. 7. The results are discussed with regard to the possible functional significance of each of the corticofugal pathways.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus K., Donate-Oliver F. Cells of origin of the occipito-pontine projection in the cat: functional properties and intracortical location. Exp Brain Res. 1977 May 23;28(1-2):167–174. doi: 10.1007/BF00237094. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. Single-unit recording from antidromically activated optic radiation neurones. J Physiol. 1962 Aug;162:432–450. doi: 10.1113/jphysiol.1962.sp006943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Blakemore C., Pettigrew J. D. The neural mechanism of binocular depth discrimination. J Physiol. 1967 Nov;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann T. P., Spear P. D. Role of the lateral suprasylvian visual area in behavioral recovery from effects of visual cortex damage in cats. Brain Res. 1977 Dec 23;138(3):445–468. doi: 10.1016/0006-8993(77)90683-7. [DOI] [PubMed] [Google Scholar]
- Berlucchi G., Rizzolatti G. Binocularly driven neurons in visual cortex of split-chiasm cats. Science. 1968 Jan 19;159(3812):308–310. doi: 10.1126/science.159.3812.308. [DOI] [PubMed] [Google Scholar]
- Bishop P. O., Henry G. H., Smith C. J. Binocular interaction fields of single units in the cat striate cortex. J Physiol. 1971 Jul;216(1):39–68. doi: 10.1113/jphysiol.1971.sp009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Henry G. H. Spatial vision. Annu Rev Psychol. 1971;22:119–160. doi: 10.1146/annurev.ps.22.020171.001003. [DOI] [PubMed] [Google Scholar]
- Blakemore C. Binocular depth discrimination and the nasotemporal division. J Physiol. 1969 Nov;205(2):471–497. doi: 10.1113/jphysiol.1969.sp008978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C. Binocular depth perception and the optic chiasm. Vision Res. 1970 Jan;10(1):43–47. doi: 10.1016/0042-6989(70)90060-x. [DOI] [PubMed] [Google Scholar]
- Bunt A. H., Minckler D. S., Johanson G. W. Demonstration of bilateral projection of the central retina of the monkey with horseradish peroxidase neuronography. J Comp Neurol. 1977 Feb 15;171(4):619–630. doi: 10.1002/cne.901710412. [DOI] [PubMed] [Google Scholar]
- CHOUDHURY B. P., WHITTERIDGE D., WILSON M. E. THE FUNCTION OF THE CALLOSAL CONNECTIONS OF THE VISUAL CORTEX. Q J Exp Physiol Cogn Med Sci. 1965 Apr;50:214–219. doi: 10.1113/expphysiol.1965.sp001783. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Morstyn R., Wagner H. G. Lateral geniculate relay of slowly conducting retinal afferents to cat visual cortex. J Physiol. 1976 Feb;255(1):299–320. doi: 10.1113/jphysiol.1976.sp011281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow B. M., Dubner R. Single-unit responses to moving visual stimuli in middle suprasylvian gyrus of the cat. J Neurophysiol. 1971 Jan;34(1):47–55. doi: 10.1152/jn.1971.34.1.47. [DOI] [PubMed] [Google Scholar]
- Dubin M. W., Cleland B. G. Organization of visual inputs to interneurons of lateral geniculate nucleus of the cat. J Neurophysiol. 1977 Mar;40(2):410–427. doi: 10.1152/jn.1977.40.2.410. [DOI] [PubMed] [Google Scholar]
- Garey L. J. A light and electron microscopic study of the visual cortex of the cat and monkey. Proc R Soc Lond B Biol Sci. 1971 Oct 12;179(1054):21–40. doi: 10.1098/rspb.1971.0079. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Jones E. G., Powell T. P. Interrelationships of striate and extrastriate cortex with the primary relay sites of the visual pathway. J Neurol Neurosurg Psychiatry. 1968 Apr;31(2):135–157. doi: 10.1136/jnnp.31.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Baker J., Mower G., Glickstein M. Corticopontine cells in area 18 of the cat. J Neurophysiol. 1978 Mar;41(2):484–495. doi: 10.1152/jn.1978.41.2.484. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Kelly J. P. The projections of cells in different layers of the cat's visual cortex. J Comp Neurol. 1975 Sep;163(1):81–105. doi: 10.1002/cne.901630106. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery R. W. Patterns of fiber degeneration in the dorsal lateral geniculate nucleus of the cat following lesions in the visual cortex. J Comp Neurol. 1967 Jul;130(3):197–221. doi: 10.1002/cne.901300303. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. R. The afferent connexions and laminar distribution of cells in area 18 of the cat. J Physiol. 1980 May;302:483–505. doi: 10.1113/jphysiol.1980.sp013257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y. Recurrent collateral inhibition of visual cortical cells projecting to superior colliculus in cats. Vision Res. 1969 Nov;9(11):1367–1380. doi: 10.1016/0042-6989(69)90073-x. [DOI] [PubMed] [Google Scholar]
- Heath C. J., Jones E. G. Connexions of area 19 and the lateral suprasylvian area of the visual cortex of the cat. Brain Res. 1970 Apr 14;19(2):302–305. doi: 10.1016/0006-8993(70)90444-0. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Harvey A. R., Lund J. S. The afferent connections and laminar distribution of cells in the cat striate cortex. J Comp Neurol. 1979 Oct 15;187(4):725–744. doi: 10.1002/cne.901870406. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Lund J. S., Harvey A. R. Cells of the striate cortex projecting to the Clare-Bishop area of the cat. Brain Res. 1978 Jul 28;151(1):154–158. doi: 10.1016/0006-8993(78)90958-7. [DOI] [PubMed] [Google Scholar]
- Henry G. H. Receptive field classes of cells in the striate cortex of the cat. Brain Res. 1977 Sep 9;133(1):1–28. doi: 10.1016/0006-8993(77)90045-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. P. Conduction velocity in pathways from retina to superior colliculus in the cat: a correlation with receptive-field properties. J Neurophysiol. 1973 May;36(3):409–424. doi: 10.1152/jn.1973.36.3.409. [DOI] [PubMed] [Google Scholar]
- Holländer H. On the origin of the corticotectal projections in the cat. Exp Brain Res. 1974;21(4):433–439. doi: 10.1007/BF00237905. [DOI] [PubMed] [Google Scholar]
- Kalil R. E., Chase R. Corticofugal influence on activity of lateral geniculate neurons in the cat. J Neurophysiol. 1970 May;33(3):459–474. doi: 10.1152/jn.1970.33.3.459. [DOI] [PubMed] [Google Scholar]
- Kawamura S., Sprague J. M., Niimi K. Corticofugal projections from the visual cortices to the thalamus, pretectum and superior colliculus in the cat. J Comp Neurol. 1974 Dec 1;158(3):339–362. doi: 10.1002/cne.901580308. [DOI] [PubMed] [Google Scholar]
- Kelly J. P., Van Essen D. C. Cell structure and function in the visual cortex of the cat. J Physiol. 1974 May;238(3):515–547. doi: 10.1113/jphysiol.1974.sp010541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy H., Magnin M. Saccadic influences on single neuron activity in the medial bank of the cat's suprasylvian sulcus (Clare Bishop area). Exp Brain Res. 1977 Mar 30;27(3-4):315–317. doi: 10.1007/BF00235506. [DOI] [PubMed] [Google Scholar]
- Kirk D. L., Levick W. R., Cleland B. G. The crossed or uncrossed destination of axons of sluggish-concentric and non-concentric cat retinal ganglion cells, with an overall synthesis of the visual field representation. Vision Res. 1976;16(3):233–236. doi: 10.1016/0042-6989(76)90104-8. [DOI] [PubMed] [Google Scholar]
- Kirk D. L., Levick W. R., Cleland B. G., Wässle H. Crossed and uncrossed representation of the visual field by brisk-sustained and brisk-transient cat retinal ganglion cells. Vision Res. 1976;16(3):225–231. doi: 10.1016/0042-6989(76)90103-6. [DOI] [PubMed] [Google Scholar]
- Leicester J. Projection of the visual vertical meridian to cerebral cortex of the cat. J Neurophysiol. 1968 May;31(3):371–382. doi: 10.1152/jn.1968.31.3.371. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Lund J. S., Henry G. H., MacQueen C. L., Harvey A. R. Anatomical organization of the primary visual cortex (area 17) of the cat. A comparison with area 17 of the macaque monkey. J Comp Neurol. 1979 Apr 15;184(4):599–618. doi: 10.1002/cne.901840402. [DOI] [PubMed] [Google Scholar]
- Magalhães-Castro H. H., Saraiva P. E., Magalhães-Castro B. Identification of corticotectal cells of the visual cortex of cats by means of horseradish peroxidase. Brain Res. 1975 Jan 17;83(3):474–479. doi: 10.1016/0006-8993(75)90838-0. [DOI] [PubMed] [Google Scholar]
- McIlwain J. T. Topographic organization and convergence in corticotectal projections from areas 17, 18, and 19 in the cat. J Neurophysiol. 1977 Mar;40(2):189–198. doi: 10.1152/jn.1977.40.2.189. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E., Blakemore C. Binocular depth perception and the corpus callosum. Vision Res. 1970 Jan;10(1):49–54. doi: 10.1016/0042-6989(70)90061-1. [DOI] [PubMed] [Google Scholar]
- Mize R. R., Murphy E. H. Alterations in receptive field properties of superior colliculus cells produced by visual cortex ablation in infant and adult cats. J Comp Neurol. 1976 Aug 1;168(3):393–424. doi: 10.1002/cne.901680306. [DOI] [PubMed] [Google Scholar]
- Nelson J. I., Kato H., Bishop P. O. Discrimination of orientation and position disparities by binocularly activated neurons in cat straite cortex. J Neurophysiol. 1977 Mar;40(2):260–283. doi: 10.1152/jn.1977.40.2.260. [DOI] [PubMed] [Google Scholar]
- Niimi K., Kawamura S., Ishimaru S. Projections of the visual cortex to the lateral geniculate and posterior thalamic nuclei in the cat. J Comp Neurol. 1971 Nov;143(3):279–312. doi: 10.1002/cne.901430303. [DOI] [PubMed] [Google Scholar]
- Nikara T., Bishop P. O., Pettigrew J. D. Analysis of retinal correspondence by studying receptive fields of binocular single units in cat striate cortex. Exp Brain Res. 1968;6(4):353–372. doi: 10.1007/BF00233184. [DOI] [PubMed] [Google Scholar]
- Norton T. T. Receptive-field properties of superior colliculus cells and development of visual behavior in kittens. J Neurophysiol. 1974 Jul;37(4):674–690. doi: 10.1152/jn.1974.37.4.674. [DOI] [PubMed] [Google Scholar]
- OTSUKA R., HASSLER R. [On the structure and segmentation of the cortical center of vision in the cat]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1962;203:212–234. doi: 10.1007/BF00352744. [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Rosenquist A. C. Visual receptive fields of single striate corical units projecting to the superior colliculus in the cat. Brain Res. 1974 Feb 15;67(1):27–42. doi: 10.1016/0006-8993(74)90295-9. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Binocular interaction on single units in cat striate cortex: simultaneous stimulation by single moving slit with receptive fields in correspondence. Exp Brain Res. 1968;6(4):391–410. doi: 10.1007/BF00233186. [DOI] [PubMed] [Google Scholar]
- Richard D., Gioanni Y., Kitsikis A., Buser P. A study of geniculate unit activity during cryogenic blockade of the primary visual cortex in the cat. Exp Brain Res. 1975 Mar 27;22(3):235–242. doi: 10.1007/BF00234766. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Tradardi V., Camarda R. Unit responses to visual stimuli in the cat's superior colliculus after removal of the visual cortex. Brain Res. 1970 Dec 1;24(2):336–339. doi: 10.1016/0006-8993(70)90114-9. [DOI] [PubMed] [Google Scholar]
- Rosenquist A. C., Palmer L. A. Visual receptive field properties of cells of the superior colliculus after cortical lesions in the cat. Exp Neurol. 1971 Dec;33(3):629–652. doi: 10.1016/0014-4886(71)90133-6. [DOI] [PubMed] [Google Scholar]
- Roucoux A., Crommelinck M. Eye movements evoked by superior colliculus stimulation in the alert cat. Brain Res. 1976 Apr 23;106(2):349–363. doi: 10.1016/0006-8993(76)91030-1. [DOI] [PubMed] [Google Scholar]
- SPRAGUE J. M., MEIKLE T. H., Jr THE ROLE OF THE SUPERIOR COLLICULUS IN VISUALLY GUIDED BEHAVIOR. Exp Neurol. 1965 Jan;11:115–146. doi: 10.1016/0014-4886(65)90026-9. [DOI] [PubMed] [Google Scholar]
- Sanderson K. J., Bishop P. O., Darian-Smith I. The properties of the binocular receptive fields of lateral geniculate neurons. Exp Brain Res. 1971;13(2):178–207. doi: 10.1007/BF00234085. [DOI] [PubMed] [Google Scholar]
- Schmielau F., Singer W. The role of visual cortex for binocular interactions in the cat lateral geniculate nucleus. Brain Res. 1977 Jan 21;120(2):354–361. doi: 10.1016/0006-8993(77)90914-3. [DOI] [PubMed] [Google Scholar]
- Shatz C. J. Anatomy of interhemispheric connections in the visual system of Boston Siamese and ordinary cats. J Comp Neurol. 1977 Jun 1;173(3):497–518. doi: 10.1002/cne.901730307. [DOI] [PubMed] [Google Scholar]
- Shoumura K. An attempt to relate the origin and distribution of commissural fibers to the presence of large and medium pyramids in layer III in the cat's visual cortex. Brain Res. 1974 Feb 15;67(1):13–25. doi: 10.1016/0006-8993(74)90294-7. [DOI] [PubMed] [Google Scholar]
- Singer W. Control of thalamic transmission by corticofugal and ascending reticular pathways in the visual system. Physiol Rev. 1977 Jul;57(3):386–420. doi: 10.1152/physrev.1977.57.3.386. [DOI] [PubMed] [Google Scholar]
- Singer W., Tretter F., Cynader M. Organization of cat striate cortex: a correlation of receptive-field properties with afferent and efferent connections. J Neurophysiol. 1975 Sep;38(5):1080–1098. doi: 10.1152/jn.1975.38.5.1080. [DOI] [PubMed] [Google Scholar]
- Sprague J. M., Levy J., DiBerardino A., Berlucchi G. Visual cortical areas mediating form discrimination in the cat. J Comp Neurol. 1977 Apr 1;172(3):441–488. doi: 10.1002/cne.901720305. [DOI] [PubMed] [Google Scholar]
- Stone J., Fukuda Y. The naso-temporal division of the cat's retina re-examined in terms of Y-, X- and W-cells. J Comp Neurol. 1974 Jun 15;155(4):377–394. doi: 10.1002/cne.901550402. [DOI] [PubMed] [Google Scholar]
- Stone J., Leicester J., Sherman S. M. The naso-temporal division of the monkey's retina. J Comp Neurol. 1973 Aug;150(3):333–348. doi: 10.1002/cne.901500306. [DOI] [PubMed] [Google Scholar]
- Toyama K., Matsunami K. Convergence of specific visual and commissural impulses upon inhibitory interneurones in cats visual cortex. Neuroscience. 1976;1(2):107–112. doi: 10.1016/0306-4522(76)90004-x. [DOI] [PubMed] [Google Scholar]
- Toyama K., Matsunami K., Ono T., Tokashiki S. An intracellular study of neuronal organization in the visual cortex. Exp Brain Res. 1974;21(1):45–66. doi: 10.1007/BF00234257. [DOI] [PubMed] [Google Scholar]
- Tretter F., Cynader M., Singer W. Cat parastriate cortex: a primary or secondary visual area. J Neurophysiol. 1975 Sep;38(5):1099–1113. doi: 10.1152/jn.1975.38.5.1099. [DOI] [PubMed] [Google Scholar]
- Tsumoto T., Creutzfeldt O. D., Legéndy C. R. Functional organization of the corticofugal system from visual cortex to lateral geniculate nucleus in the cat (with an appendix on geniculo-cortical mono-synaptic connections). Exp Brain Res. 1978 Jul 14;32(3):345–364. doi: 10.1007/BF00238707. [DOI] [PubMed] [Google Scholar]
- Tusa R. J., Palmer L. A., Rosenquist A. C. The retinotopic organization of area 17 (striate cortex) in the cat. J Comp Neurol. 1978 Jan 15;177(2):213–235. doi: 10.1002/cne.901770204. [DOI] [PubMed] [Google Scholar]
- Tömböl T., Hajdu F., Somogyi G. Identification of the Golgi picture of the layer VI cortic-geniculate projection neurons. Exp Brain Res. 1975 Nov 28;24(1):107–110. doi: 10.1007/BF00236022. [DOI] [PubMed] [Google Scholar]
- Updyke B. V. The patterns of projection of cortical areas 17, 18, and 19 onto the laminae of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol. 1975 Oct 15;163(4):377–395. doi: 10.1002/cne.901630402. [DOI] [PubMed] [Google Scholar]
- Updyke B. V. Topographic organization of the projections from cortical areas 17, 18 and 19 onto the thalamus, pretectum and superior colliculus in the cat. J Comp Neurol. 1977 May 1;173(1):81–122. doi: 10.1002/cne.901730106. [DOI] [PubMed] [Google Scholar]
- Vesbaesya C., Whitteridge D., Wilson M. E. Callosal connexions of the cortex representing the area centralis. J Physiol. 1967 Jul;191(2):79P–80P. [PubMed] [Google Scholar]
- Westheimer G., Mitchell D. E. The sensory stimulus for disjunctive eye movements. Vision Res. 1969 Jul;9(7):749–755. doi: 10.1016/0042-6989(69)90012-1. [DOI] [PubMed] [Google Scholar]
- Wickelgren B. G., Sterling P. Influence of visual cortex on receptive fields in the superior colliculus of the cat. J Neurophysiol. 1969 Jan;32(1):16–23. doi: 10.1152/jn.1969.32.1.16. [DOI] [PubMed] [Google Scholar]
- Wilson M. E. Cortico-cortical connexions of the cat visual areas. J Anat. 1968 Mar;102(Pt 3):375–386. [PMC free article] [PubMed] [Google Scholar]