Abstract
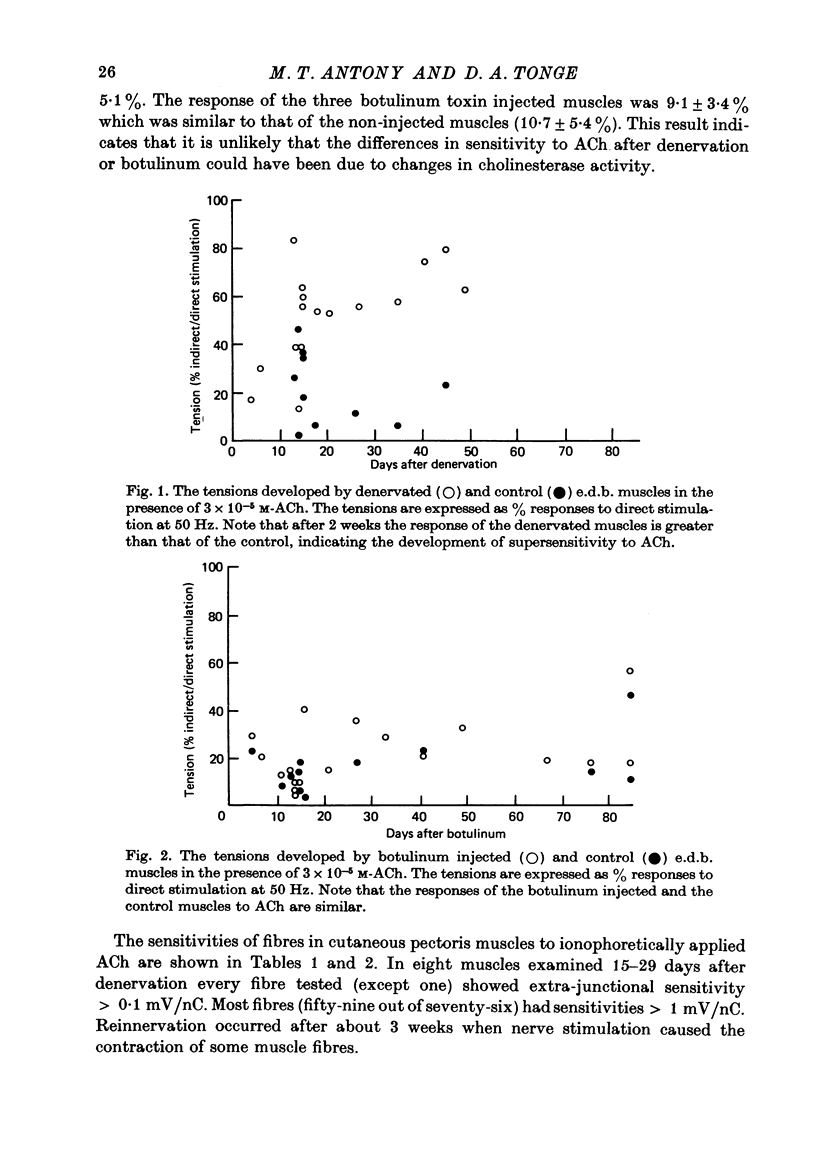
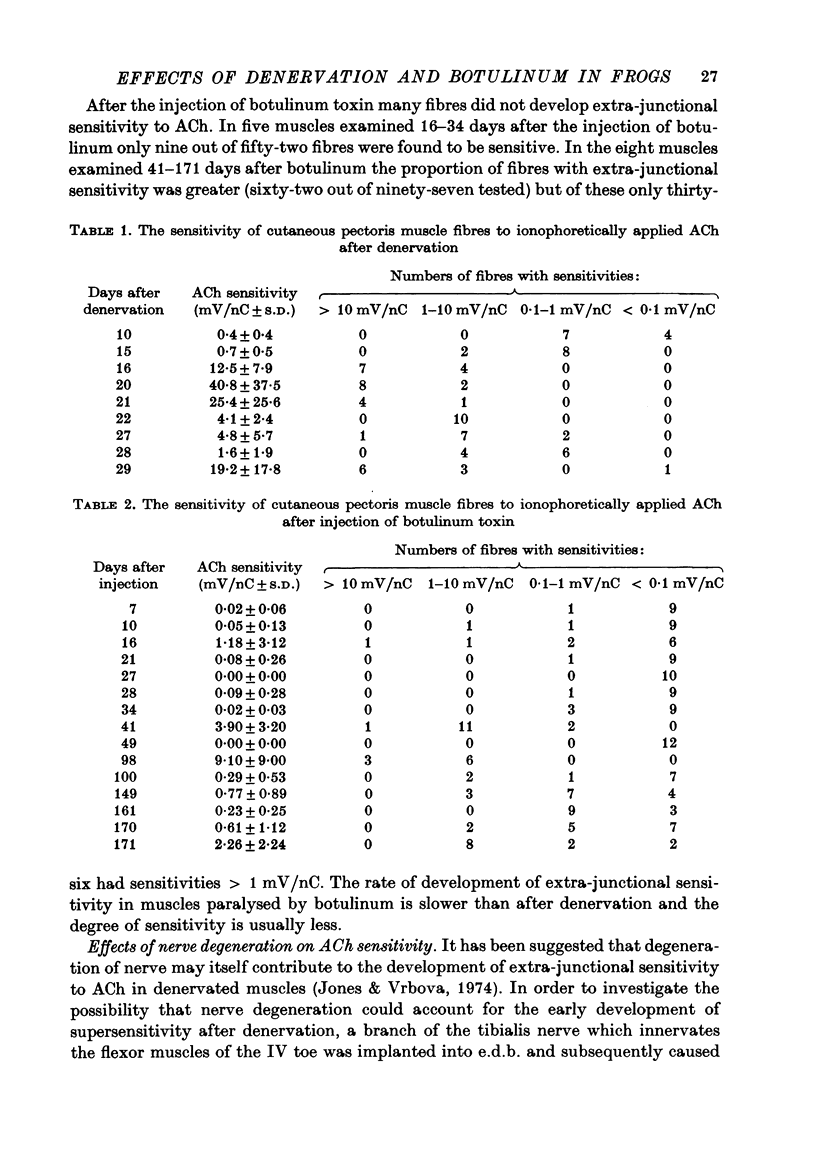
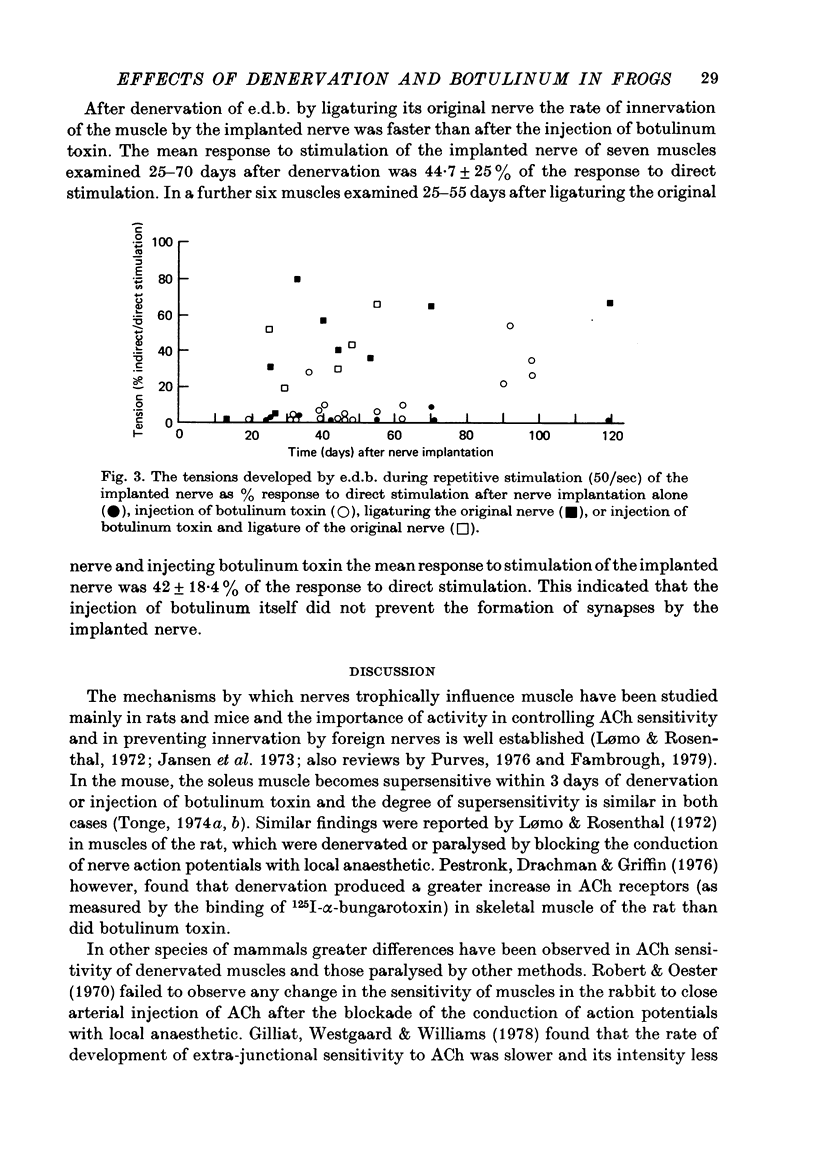
1. The effects of denervation and local paralysis produced by botulinum toxin (type D) on the sensitivity of skeletal muscle to ACh and its ability to accept innervation by an implanted foreign nerve were investigated in the frog. 2. Denervated muscles developed supersensitivity to ACh within 2 weeks and became extensively innervated by an implanted foreign nerve after about 4 weeks. 3. Chronic electrical stimulation of denervated muscles (50 Hz for 1 sec every 60 sec) did not prevent the development of supersensitivity. 4. Muscles paralysed by botulinum toxin did not usually develop supersensitivity to ACh until after 2-3 months and the extra-junctional sensitivity of individual fibres was generally less than after denervation. Significant innervation of the paralysed muscles by an implanted foreign nerve did not occur until after 2-3 months. 5. The results suggest that in the frog nerves are able to control muscle sensitivity to ACh and to prevent innervation by foreign nerves by some mechanism other than muscle activity. Prolonged inactivity seems to result in some development of extra-junctional sensitivity and acceptance of foreign innervation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGEN A. S. V., DICKENS F., ZATMAN L. J. The action of botulinum toxin on the neuro-muscular junction. J Physiol. 1949 Aug;109(1-2):10–24. doi: 10.1113/jphysiol.1949.sp004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. K., Hall Z. W. Increased extrajunctional acetylcholine sensitivity produced by chronic acetylcholine sensitivity produced by chronic post-synaptic neuromuscular blockade. J Physiol. 1975 Jan;244(3):659–676. doi: 10.1113/jphysiol.1975.sp010818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Sargent P. B. Loss of extrasynaptic acetylcholine sensitivity upon reinnervation of parasympathetic ganglion cells. J Physiol. 1979 Apr;289:263–275. doi: 10.1113/jphysiol.1979.sp012736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K. The spread of acetylcholine sensitivity after denervation of frog skeletal muscle fibers. Pflugers Arch. 1974 May 6;348(4):287–292. doi: 10.1007/BF00589218. [DOI] [PubMed] [Google Scholar]
- Duchen L. W. An electron microscopic study of the changes induced by botulinum toxin in the motor end-plates of slow and fast skeletal muscle fibres of the mouse. J Neurol Sci. 1971 Sep;14(1):47–60. doi: 10.1016/0022-510x(71)90129-8. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Fex S., Sonesson B., Thesleff S., Zelená J. Nerve implants in botulinum poisoned mammalian muscle. J Physiol. 1966 Jun;184(4):872–882. doi: 10.1113/jphysiol.1966.sp007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliatt R. W., Westgaard R. H., Williams I. R. Extrajunctional acetylcholine sensitivity of inactive muscle fibres in the baboon during prolonged nerve pressure block. J Physiol. 1978 Jul;280:499–514. doi: 10.1113/jphysiol.1978.sp012397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell A. D., Letinsky M. S., Rheuben M. B. Competitive interaction between foreign nerves innervating frog skeletal muscle. J Physiol. 1979 Apr;289:241–262. doi: 10.1113/jphysiol.1979.sp012735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J. K., Lomo T., Nicolaysen K., Westgaard R. H. Hyperinnervation of skeletal muscle fibers: dependence on muscle activity. Science. 1973 Aug 10;181(4099):559–561. doi: 10.1126/science.181.4099.559. [DOI] [PubMed] [Google Scholar]
- Jones R., Vrbová G. Two factors responsible for the development of denervation hypersensitivity. J Physiol. 1974 Feb;236(3):517–538. doi: 10.1113/jphysiol.1974.sp010450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Further studies on the control of ACh sensitivity by muscle activity in the rat. J Physiol. 1975 Nov;252(3):603–626. doi: 10.1113/jphysiol.1975.sp011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. FORMATION OF EXTRA NERVE-MUSCLE JUNCTIONS IN INNERVATED MUSCLE. Nature. 1963 Sep 21;199:1191–1192. doi: 10.1038/1991191a0. [DOI] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Spitzer N. C. Absence of action potentials in frog slow muscle fibres paralysed by botulinum toxin. J Physiol. 1974 Aug;241(1):183–199. doi: 10.1113/jphysiol.1974.sp010648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasledov G. A., Thesleff S. Denervation changes in frog skeletal muscle. Acta Physiol Scand. 1974 Feb;90(2):370–380. doi: 10.1111/j.1748-1716.1974.tb05598.x. [DOI] [PubMed] [Google Scholar]
- Pestronk A., Drachman D. B., Griffin J. W. Effect of botulinum toxin on trophic regulation of acetylcholine receptors. Nature. 1976 Dec 23;264(5588):787–789. doi: 10.1038/264787a0. [DOI] [PubMed] [Google Scholar]
- Robert E. D., Oester Y. T. Absence of supersensitivity to acetylcholine in innervated muscle subjected to a prolonged pharmacologic nerve block. J Pharmacol Exp Ther. 1970 Jul;174(1):133–140. [PubMed] [Google Scholar]
- Schmidt H., Stefani E. Action potentials in slow muscle fibres of the frog during regeneration of motor nerves. J Physiol. 1977 Sep;270(2):507–517. doi: 10.1113/jphysiol.1977.sp011965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THESLEFF S. Supersensitivity of skeletal muscle produced by botulinum toxin. J Physiol. 1960 Jun;151:598–607. doi: 10.1113/jphysiol.1960.sp006463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge D. A. Chronic effects of botulinum toxin on neuromuscular transmission and sensitivity to acetylcholine in slow and fast skeletal muscle of the mouse. J Physiol. 1974 Aug;241(1):127–139. doi: 10.1113/jphysiol.1974.sp010644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge D. A. Effect of implantation of an extra nerve on the recovery of neuromuscular transmission from botulinum toxin. J Physiol. 1977 Mar;265(3):809–820. doi: 10.1113/jphysiol.1977.sp011745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge D. A. Physiological characteristics of re-innervation of skeletal muscle in the mouse. J Physiol. 1974 Aug;241(1):141–153. doi: 10.1113/jphysiol.1974.sp010645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge D. A. Prolonged effects of a post-synaptic blocking fraction of Naja siamensis venom of skeletal muscle of the mouse. Q J Exp Physiol Cogn Med Sci. 1978 Jan;63(1):39–47. doi: 10.1113/expphysiol.1978.sp002413. [DOI] [PubMed] [Google Scholar]


