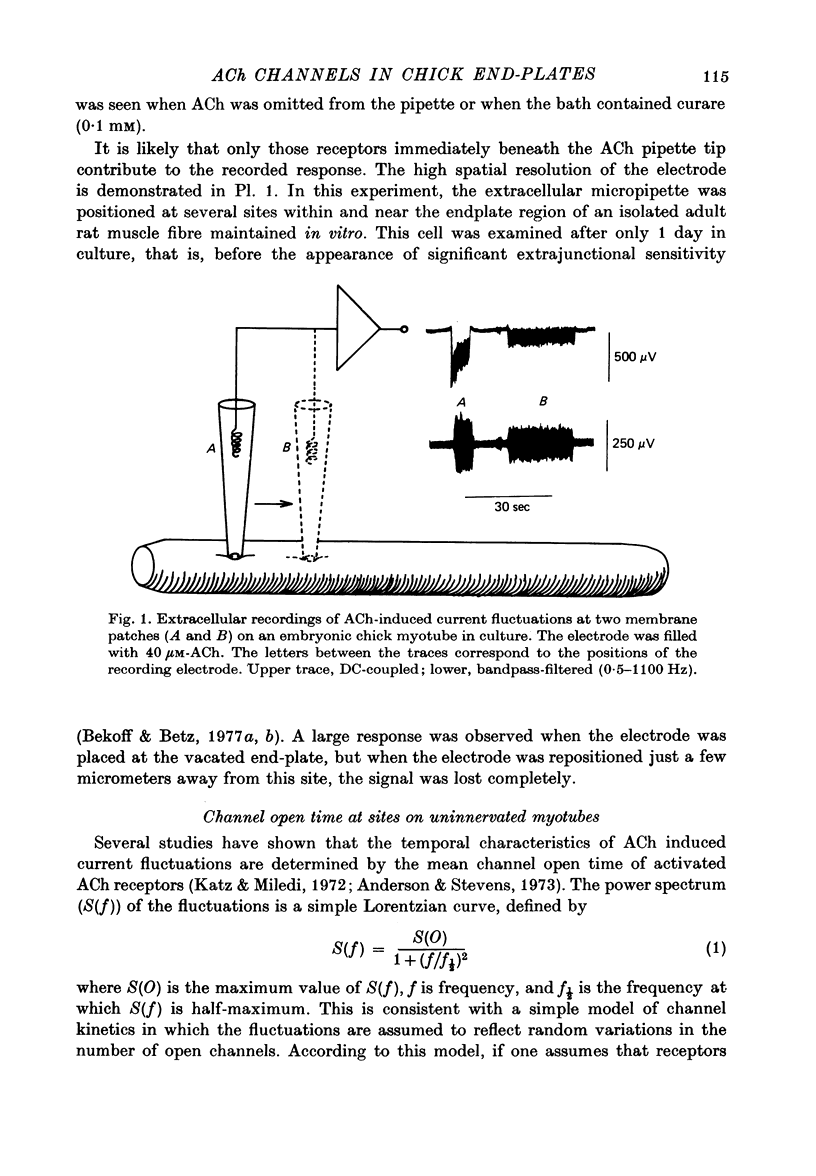
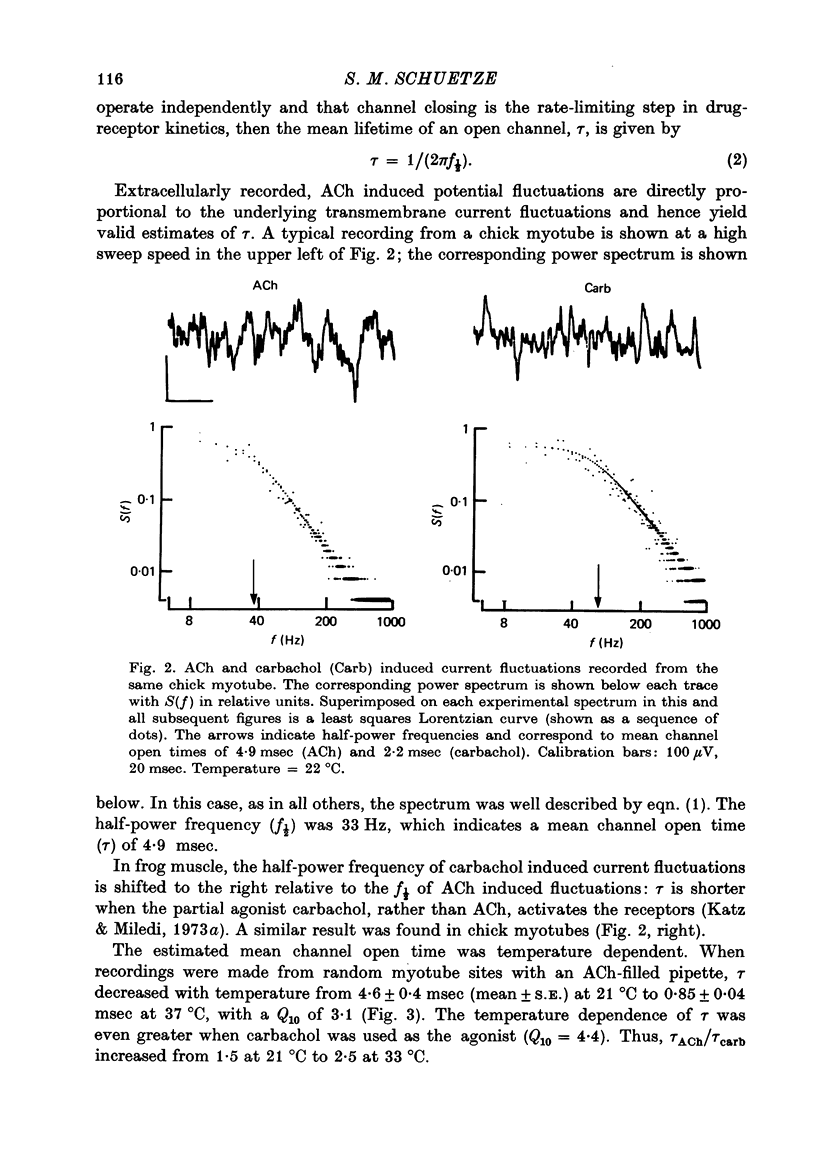
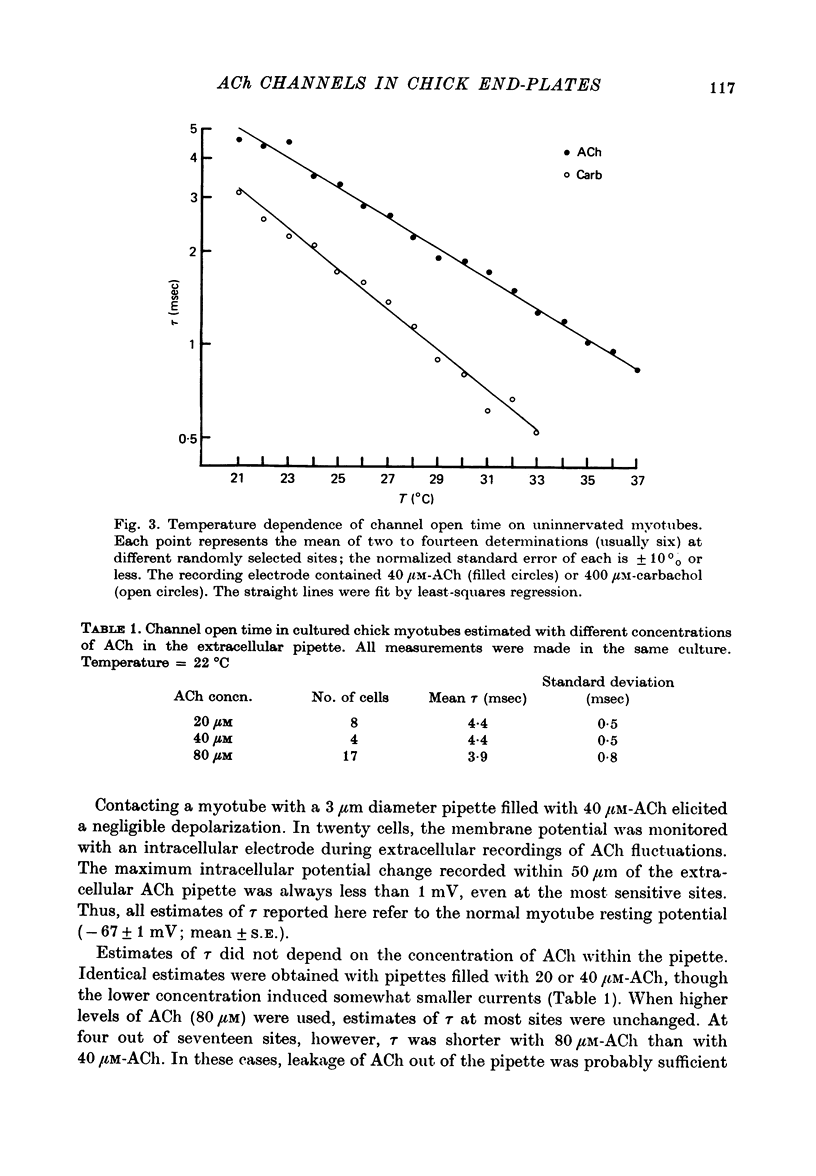
Abstract
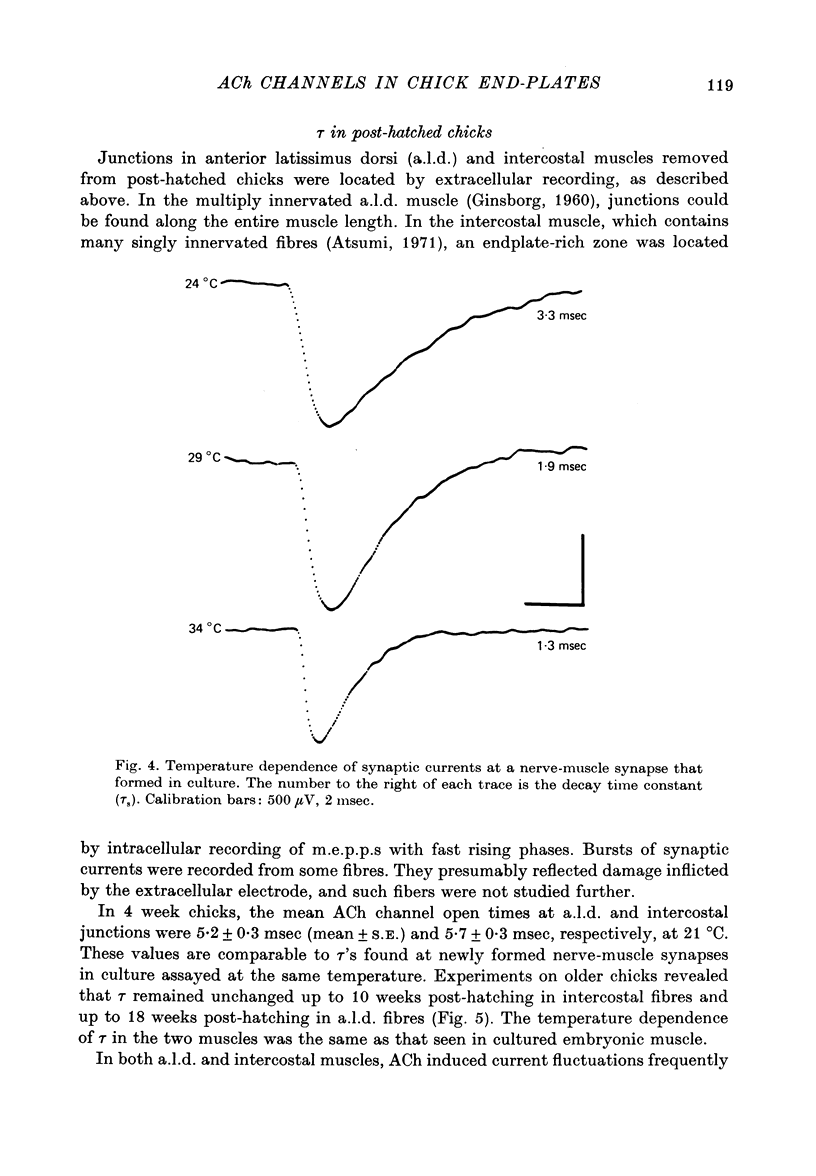
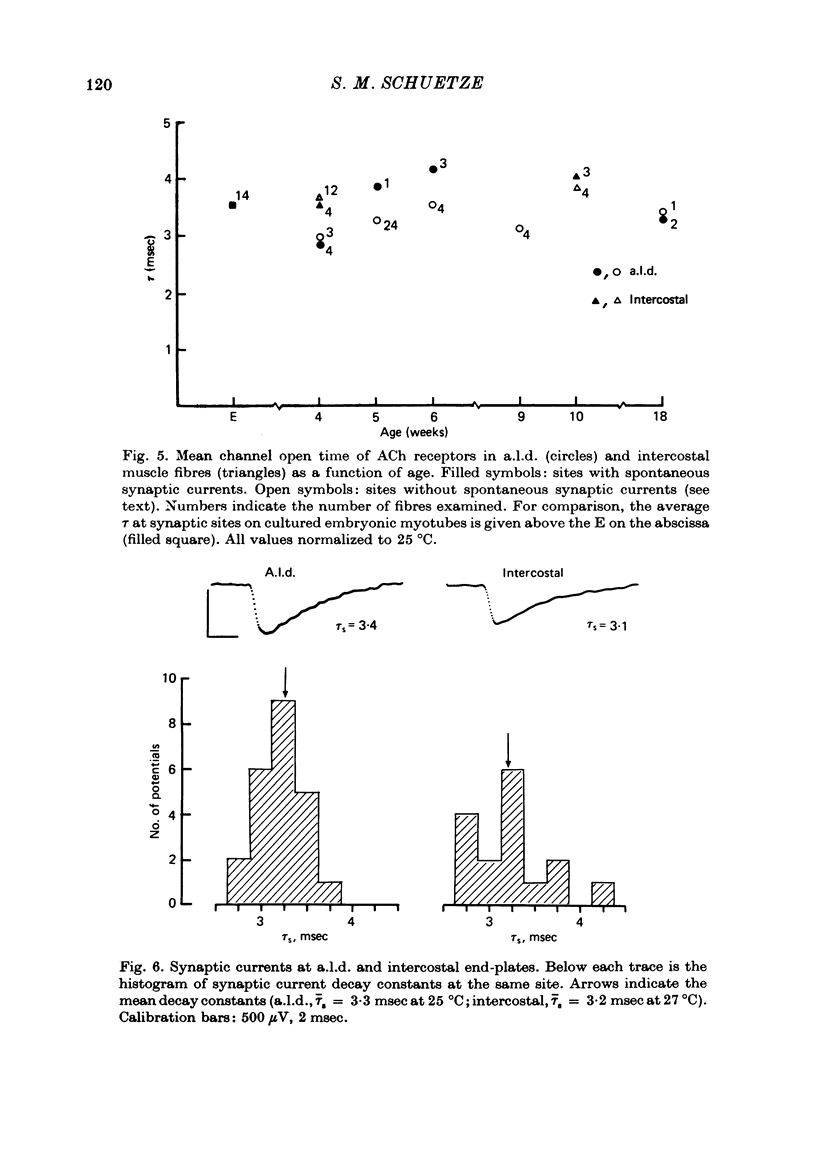
1. The mean channel open time (tau) of ACh receptors was measured in chick muscles at various stages of development. Tau was estimated by analysing ACh induced current fluctuations recorded extracellularly from small (ca. 20 micrometers2) membrane patches. 2. At random sites on uninnervated, embryonic chick muscle fibres in vitro, tau was relatively long--4 msec at 23 degrees C. 3. Estimates of tau at synaptic sites on embryonic myotubes innervated in vitro were identical to estimates at extrasynaptic sites on the same fibres. Both were comparable to estimates on uninnervated myotubes. 4. Synaptic currents at cultured junctions decayed slowly as simple exponentials. The decay time constants were never shorter than the mean channel open time measured by fluctuation analysis. 5. In anterior latissimus dorsi and intercostal muscle fibres of 4- to 18-week posthatched chicks, fluctuation analysis and synaptic current decays indicate that the channel open time of mature chick endplate receptors is as long as that of embryonic synaptic receptors in vitro. Apparently, tau remains prolonged throughout the maturation of chick neuromuscular junctions.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsumi S. The histogenesis of motor neurons with special reference to the correlation of their endplate formation. I. The development of endplates in the intercostal muscle in the chick embryo. Acta Anat (Basel) 1971;80(2):161–182. doi: 10.1159/000143687. [DOI] [PubMed] [Google Scholar]
- Bekoff A., Betz W. J. Physiological properties of dissociated muscle fibres obtained from innervated and denervated adult rat muscle. J Physiol. 1977 Sep;271(1):25–40. doi: 10.1113/jphysiol.1977.sp011988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekoff A., Betz W. Properties of isolated adult rat muscle fibres maintained in tissue culture. J Physiol. 1977 Oct;271(2):537–547. doi: 10.1113/jphysiol.1977.sp012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden S. Acetylcholine receptors at the neuromuscular junction: developmental change in receptor turnover. Dev Biol. 1977 Nov;61(1):79–85. doi: 10.1016/0012-1606(77)90343-8. [DOI] [PubMed] [Google Scholar]
- Cohen S. A., Fischbach G. D. Clusters of acetylcholine receptors located at identified nerve-muscle synapses in vitro. Dev Biol. 1977 Aug;59(1):24–38. doi: 10.1016/0012-1606(77)90237-8. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Large W. A., Rang H. P. An analysis of the action of a false transmitter at the neuromuscular junction. J Physiol. 1977 Apr;266(2):361–395. doi: 10.1113/jphysiol.1977.sp011772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Trautmann A. Acetylcholine-induced channels and transmitter release at human endplates. Nature. 1978 Jan 5;271(5640):74–75. doi: 10.1038/271074a0. [DOI] [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Acetylcholine receptor turnover in membranes of developing muscle fibers. J Cell Biol. 1975 May;65(2):335–358. doi: 10.1083/jcb.65.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Nameroff M., Nelson P. G. Electrical properties of chick skeletal muscle fibers developing in cell culture. J Cell Physiol. 1971 Oct;78(2):289–299. doi: 10.1002/jcp.1040780218. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Schuetze S. M. A post-natal decrease in acetylcholine channel open time at rat end-plates. J Physiol. 1980 Jun;303:125–137. doi: 10.1113/jphysiol.1980.sp013275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D. Synapse formation between dissociated nerve and muscle cells in low density cell cultures. Dev Biol. 1972 Jun;28(2):407–429. doi: 10.1016/0012-1606(72)90023-1. [DOI] [PubMed] [Google Scholar]
- Frank E., Fischbach G. D. ACh receptors accumulate at newly formed nerve-muscle synapses in vitro. Soc Gen Physiol Ser. 1977;32:285–291. [PubMed] [Google Scholar]
- Frank E., Fischbach G. D. Early events in neuromuscular junction formation in vitro: induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J Cell Biol. 1979 Oct;83(1):143–158. doi: 10.1083/jcb.83.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBORG B. L. Some properties of avian skeletal muscle fibres with multiple neuromuscular junctions. J Physiol. 1960 Dec;154:581–598. doi: 10.1113/jphysiol.1960.sp006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The characteristics of 'end-plate noise' produced by different depolarizing drugs. J Physiol. 1973 May;230(3):707–717. doi: 10.1113/jphysiol.1973.sp010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Morris D. G. The development of functional innervation in the hind limb of the chick embryo. J Physiol. 1975 Jul;249(2):301–326. doi: 10.1113/jphysiol.1975.sp011017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass Y., Fischbach G. D. A discontinuous relationship between the acetylcholine-activated channel conductance and temperature. Nature. 1976 Sep 9;263(5573):150–151. doi: 10.1038/263150a0. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Noise analysis of drug induced voltage clamp currents in denervated frog muscle fibres. J Physiol. 1976 Jul;258(3):705–729. doi: 10.1113/jphysiol.1976.sp011442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Rubin L. L., Schuetze S. M., Fischbach G. D. Accumulation of acetylcholinesterase at newly formed nerve--muscle synapases. Dev Biol. 1979 Mar;69(1):46–58. doi: 10.1016/0012-1606(79)90273-2. [DOI] [PubMed] [Google Scholar]
- Sachs F., Lecar H. Acetylcholine noise in tissue culture muscle cells. Nat New Biol. 1973 Dec 19;246(155):214–216. doi: 10.1038/newbio246214a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B. Acetylcholine-induced ionic channels in rat skeletal muscle. Fed Proc. 1978 Oct;37(12):2654–2659. [PubMed] [Google Scholar]
- Schuetze S. M., Frank E. F., Fischbach G. D. Channel open time and metabolic stability of synaptic and extrasynaptic acetylcholine receptors on cultured chick myotubes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):520–523. doi: 10.1073/pnas.75.1.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytkowski A. J., Vogel Z., Nirenberg M. W. Development of acetylcholine receptor clusters on cultured muscle cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):270–274. doi: 10.1073/pnas.70.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]