Abstract
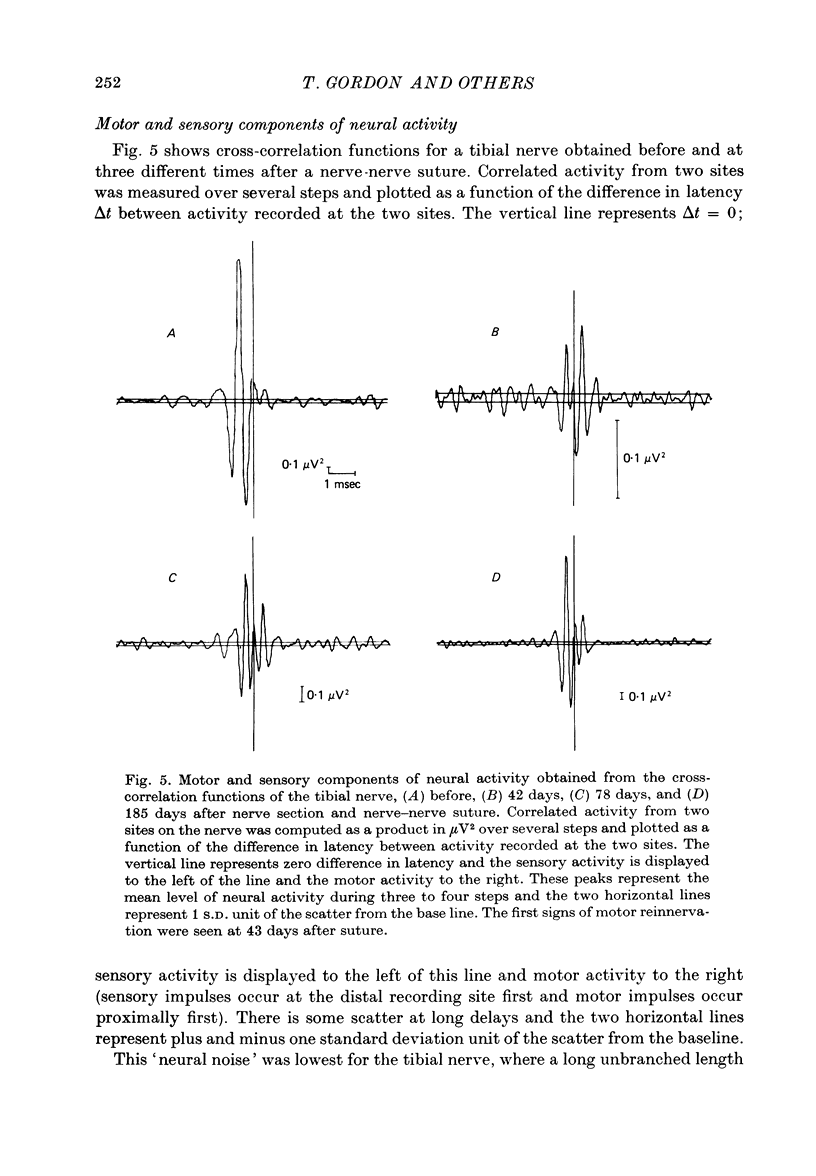
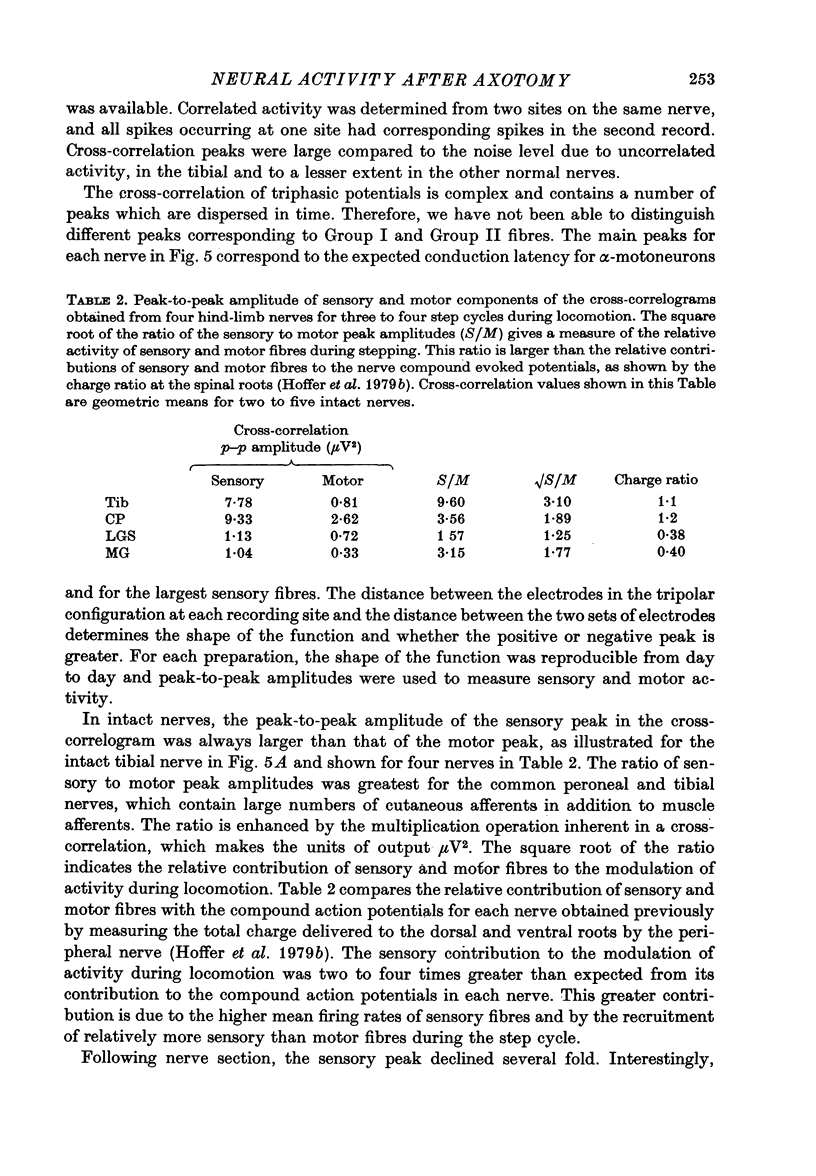
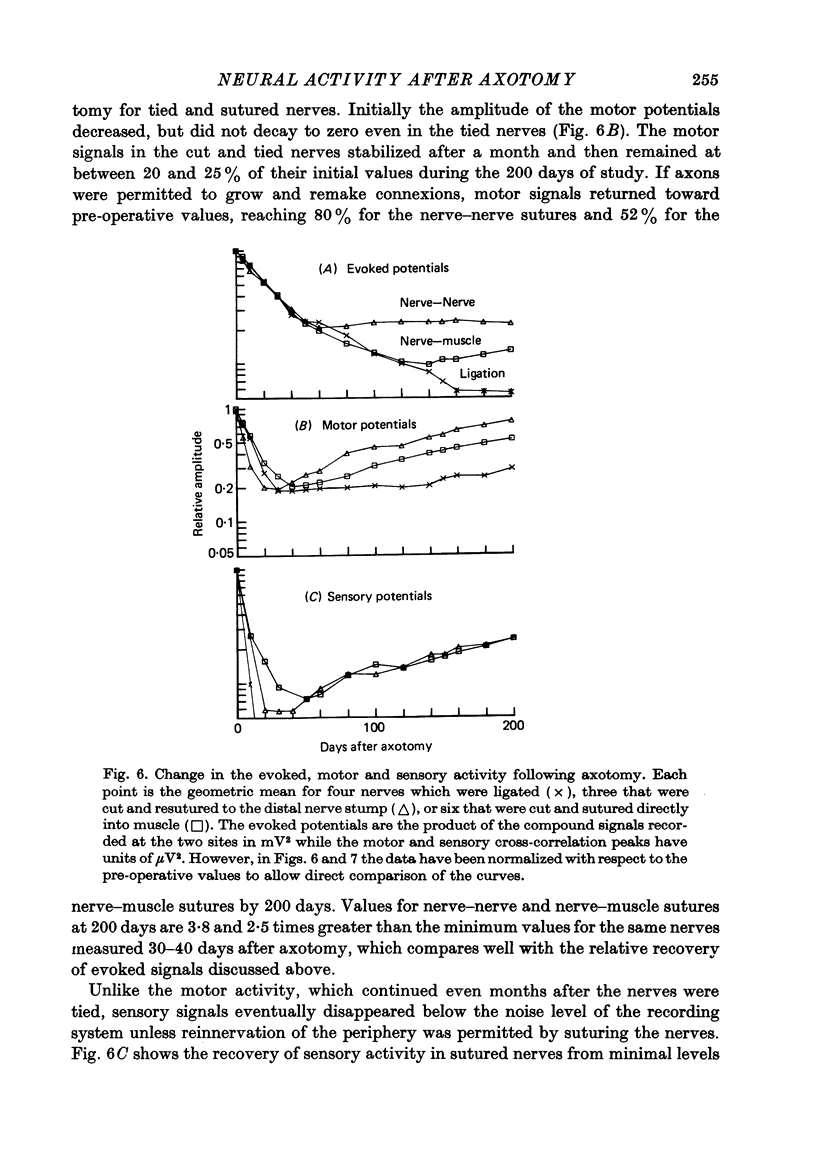
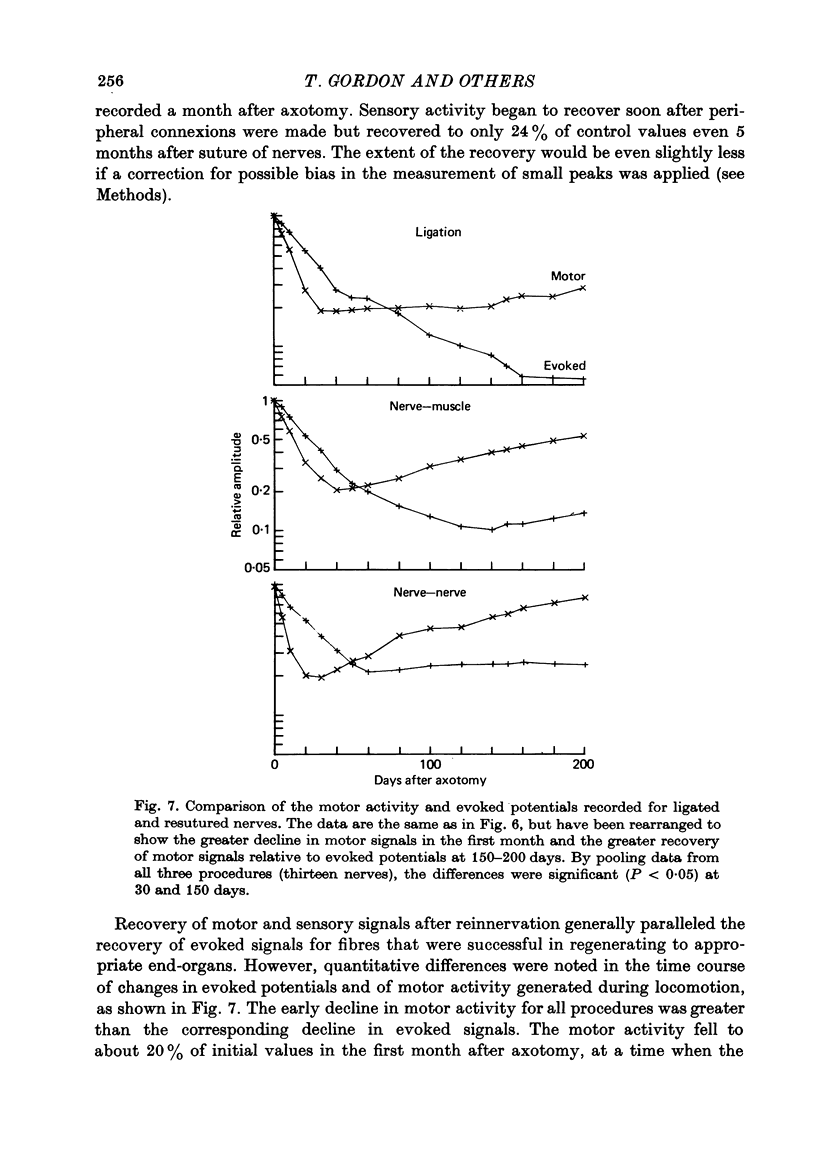
1. Neural activity was recorded from cats during locomotion on a treadmill using electrodes in Silastic cuffs placed around the sciatic nerve and the lateral gastrocnemius-soleus, medial gastrocnemius, common peroneal and tibial nerve branches. Each branch gave characteristic patterns of activity which were studied before and after it was cut distal to the recording cuffs. Sensory and motor components were separated and measured using cross-correlation techniques. The amplitude of the cross-correlation peaks was compared with the amplitude of compound action potentials evoked by electrical stimulation and recorded from the same sites in the anaesthetized animal. 2. Sensory activity declined rapidly following axotomy and did not recover unless reinnervation occurred. Sensory activity even 5 months after nerve section and resuture had recovered to only a fraction of the control values. This reduction is attributed to a decline in the evoked compound potentials and to many fibres being unsuccessful in regenerating to appropriate sensory organs. 3. Motor activity declined more than could be accounted for by a decline in evoked potentials over the first month after axotomy. The extra reduction represents a decline in the number of impulses generated by alpha-motoneurones after axotomy. If regeneration was permitted, motor activity recovered to higher levels than did the evoked potentials for the whole nerve. Even if regeneration was prevented by nerve ligation, motoneurones continued to generate activity at a stable level over a period of months during which whole nerve compound potentials continued to decline. 4. The modulation of motor activity in ligated nerves during the step cycle was still appropriate to the required movement. Thus, activity recorded from severed nerves in human amputees may be useful in controlling powered artificial limbs. The persistence of motor activity may be responsible for the lesser degree of atrophy found in motor fibres than in sensory fibres following ligation (Hoffer, Stein & Gordon, 1979b).
Full text
PDF



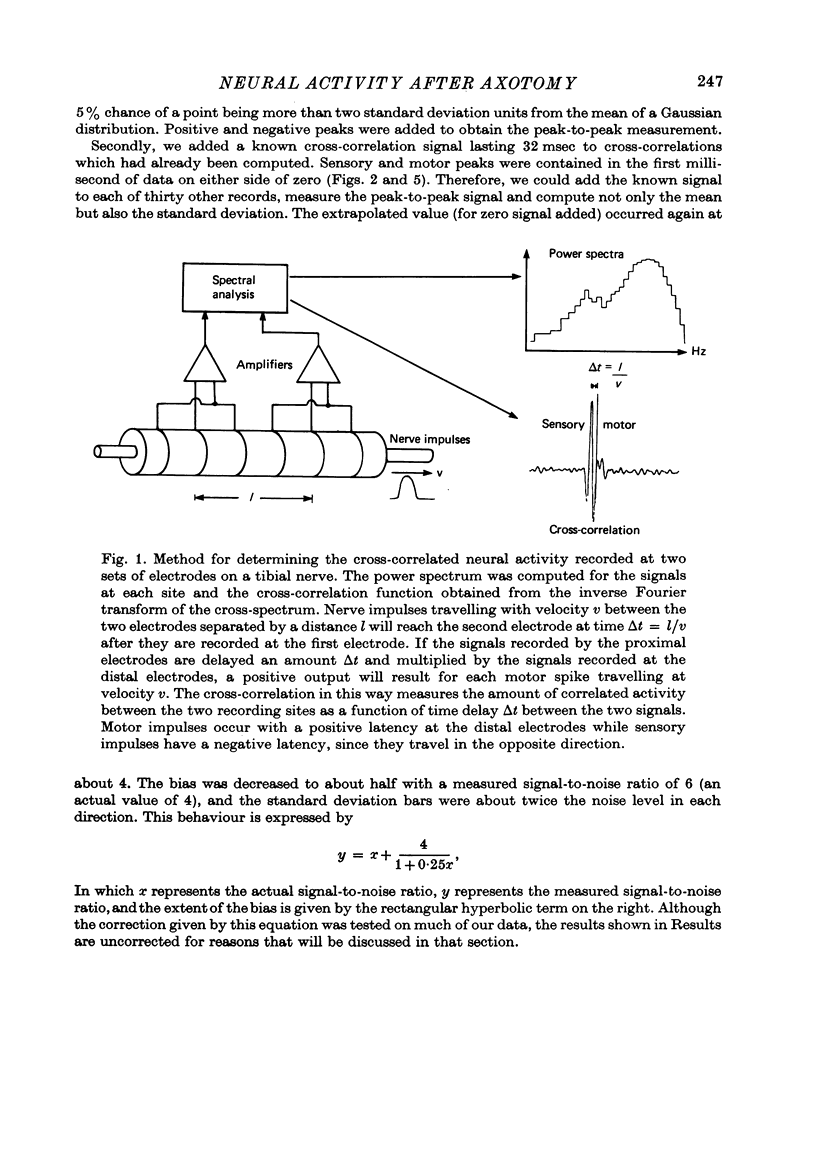
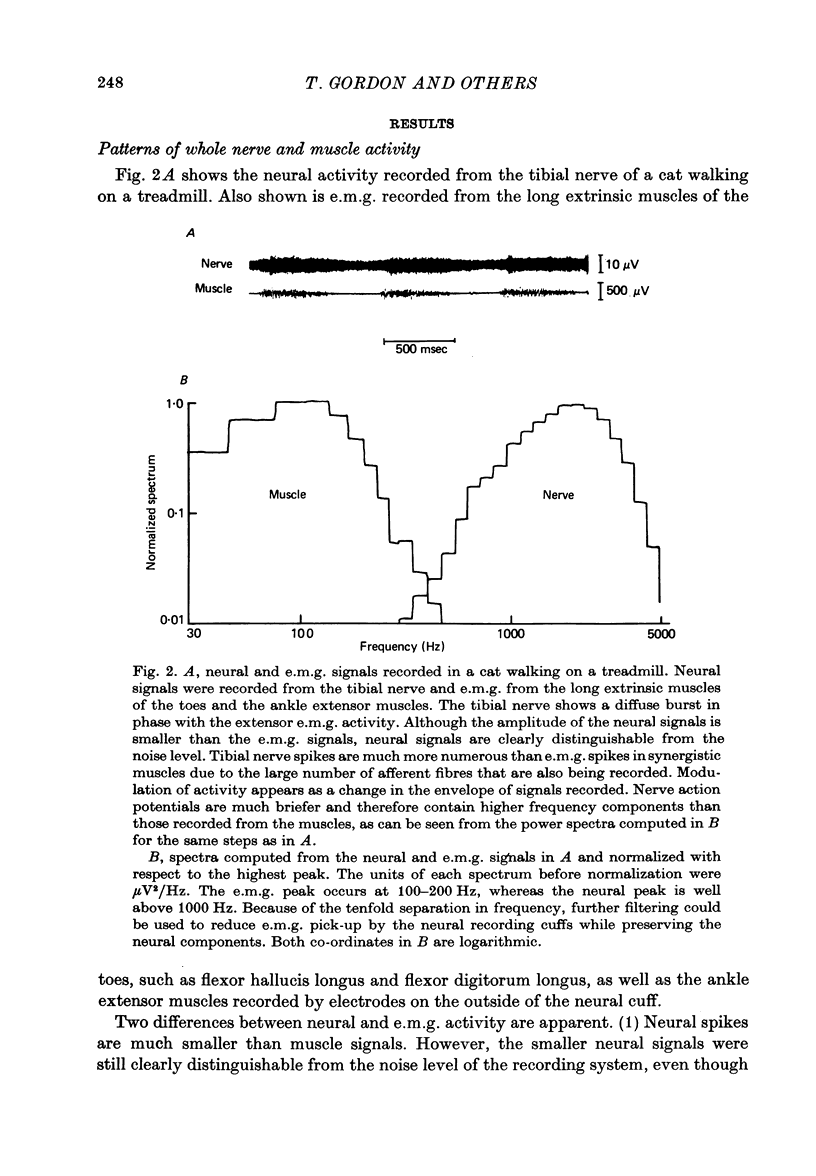
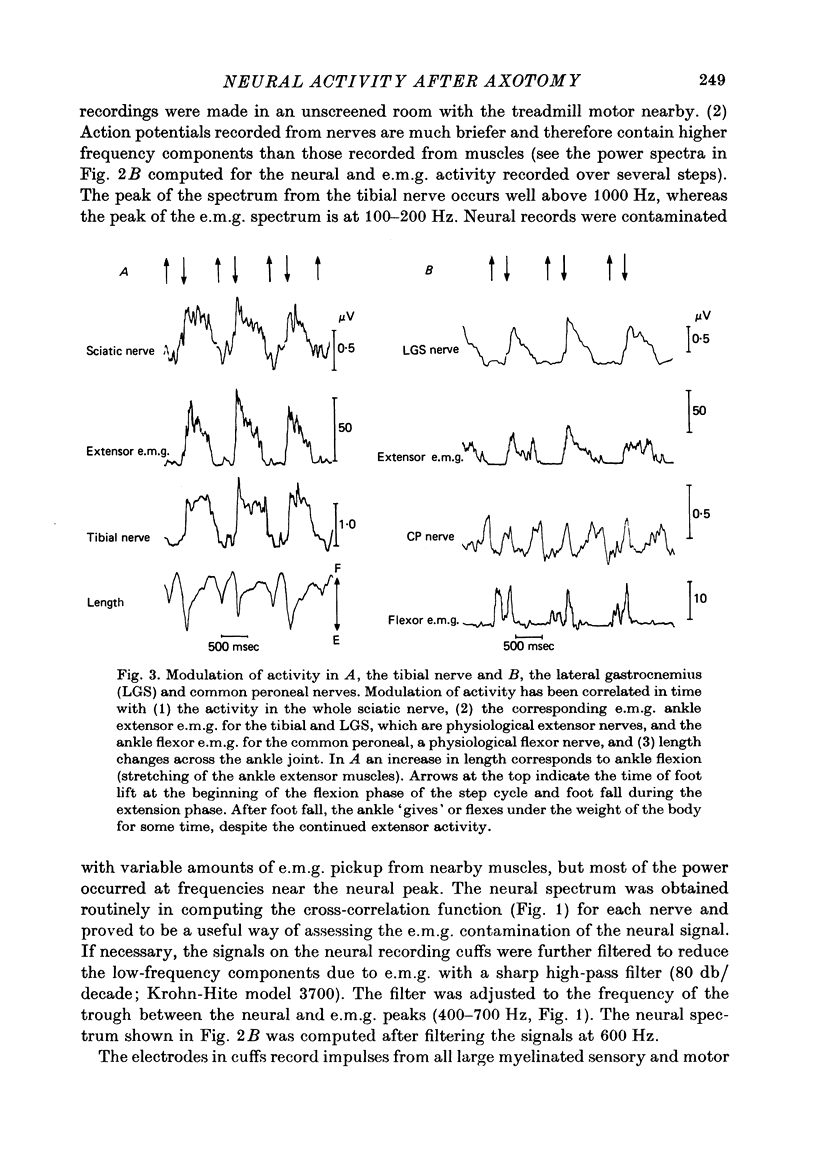

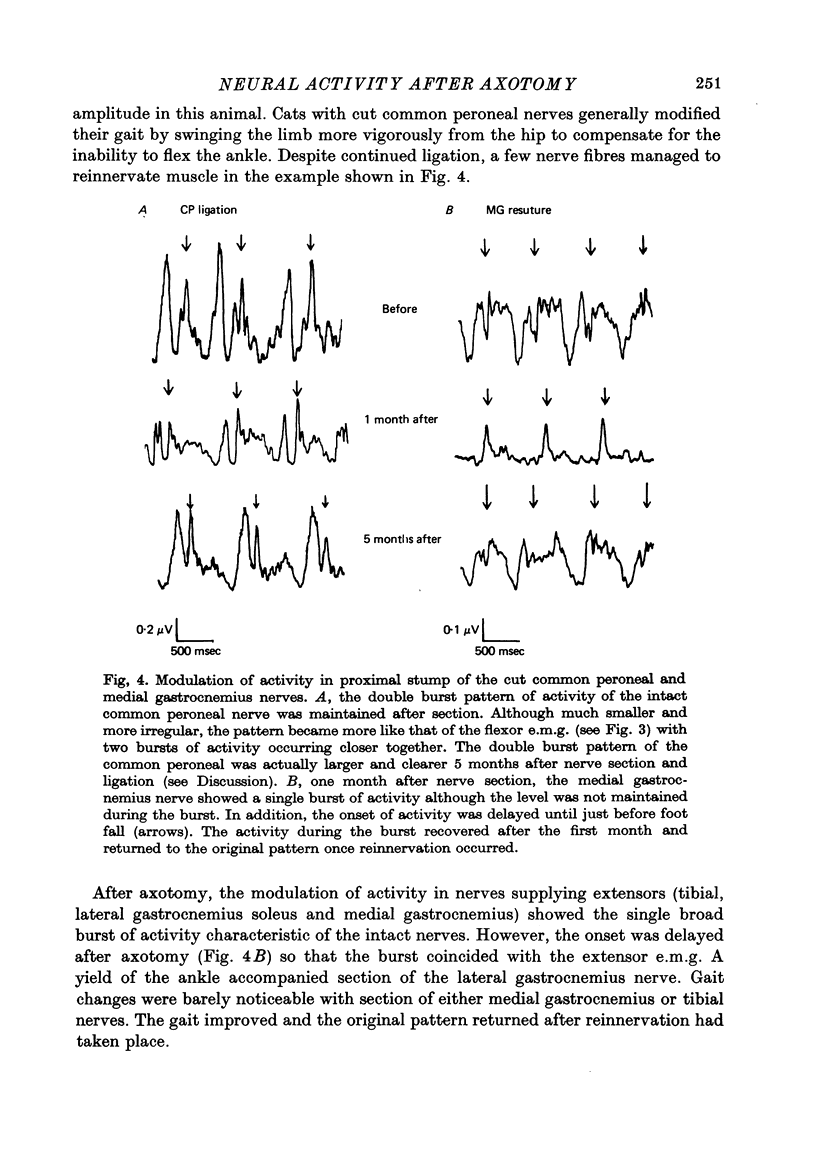








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken J. T., Sharman M., Young J. Z. Maturation of regenerating nerve fibres with various peripheral connexions. J Anat. 1947 Jan;81(Pt 1):1–22.2. [PMC free article] [PubMed] [Google Scholar]
- Blinzinger K., Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85(2):145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- CERF J. A., CHACKO L. W. Retrograde reaction in motoneuron dendrites following ventral root section in the frog. J Comp Neurol. 1958 Apr;109(2):205–219. doi: 10.1002/cne.901090205. [DOI] [PubMed] [Google Scholar]
- CRAGG B. G., THOMAS P. K. Changes in conduction velocity and fibre size proximal to peripheral nerve lesions. J Physiol. 1961 Jul;157:315–327. doi: 10.1113/jphysiol.1961.sp006724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg B. G. What is the signal for chromatolysis? Brain Res. 1970 Sep 29;23(1):1–21. doi: 10.1016/0006-8993(70)90345-8. [DOI] [PubMed] [Google Scholar]
- Cull R. E. Rôle of nerve-muscle contact in maintaining synaptic connections. Exp Brain Res. 1974;20(3):307–310. doi: 10.1007/BF00238321. [DOI] [PubMed] [Google Scholar]
- Czéh G., Kudo N., Kuno M. Membrane properties and conduction velocity in sensory neurones following central or peripheral axotomy. J Physiol. 1977 Aug;270(1):165–180. doi: 10.1113/jphysiol.1977.sp011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. A., Gordon T., Hoffer J. A., Jhamandas J., Stein R. B. Compound action potentials recorded from mammalian peripheral nerves following ligation or resuturing. J Physiol. 1978 Dec;285:543–559. doi: 10.1113/jphysiol.1978.sp012588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca C. J., Gilmore L. D. Voluntary nerve signals from severed mammalian nerves: long-term recordings. Science. 1976 Jan 16;191(4223):193–195. doi: 10.1126/science.1246608. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., LIBET B., YOUNG R. R. The behaviour of chromatolysed motoneurones studied by intracellular recording. J Physiol. 1958 Aug 29;143(1):11–40. doi: 10.1113/jphysiol.1958.sp006041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen A. A., Carpenter S., Karpati G., Bellavance A. The effect of muscle hyper- and hypoactivity upon fibre diameters of intact and regenerating nerves. J Neurol Sci. 1973 Dec;20(4):457–469. doi: 10.1016/0022-510x(73)90176-7. [DOI] [PubMed] [Google Scholar]
- Engberg I., Lundberg A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiol Scand. 1969 Apr;75(4):614–630. doi: 10.1111/j.1748-1716.1969.tb04415.x. [DOI] [PubMed] [Google Scholar]
- French A. S. Averaging of neurophysiological data prior to frequency domain analysis. Comput Programs Biomed. 1973 Oct;3(3):113–122. doi: 10.1016/0010-468x(73)90016-0. [DOI] [PubMed] [Google Scholar]
- Gordon T., Hoffer J. A., Stein R. B. Continued generation of action potentials in nerves without peripheral connexions [proceedings]. J Physiol. 1977 Oct;272(1):39P–40P. [PubMed] [Google Scholar]
- Gottlieb G. L., Agarwal G. C. Filtering of electromyographic signals. Am J Phys Med. 1970 Apr;49(2):142–146. [PubMed] [Google Scholar]
- Govrin-Lippmann R., Devor M. Ongoing activity in severed nerves: source and variation with time. Brain Res. 1978 Dec 29;159(2):406–410. doi: 10.1016/0006-8993(78)90548-6. [DOI] [PubMed] [Google Scholar]
- Gutmann E., Sanders F. K. Recovery of fibre numbers and diameters in the regeneration of peripheral nerves. J Physiol. 1943 Mar 25;101(4):489–518. doi: 10.1113/jphysiol.1943.sp004002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer J. A., Stein R. B., Gordon T. Differential atrophy of sensory and motor fibers following section of cat peripheral nerves. Brain Res. 1979 Dec 14;178(2-3):347–361. doi: 10.1016/0006-8993(79)90698-x. [DOI] [PubMed] [Google Scholar]
- Kuno M., Llinás R. Alterations of synaptic action in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):823–838. doi: 10.1113/jphysiol.1970.sp009244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Llinás R. Enhancement of synaptic transmission by dendritic potentials in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):807–821. doi: 10.1113/jphysiol.1970.sp009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Miyata Y., Muñoz-Martinez E. J. Differential reaction of fast and slow alpha-motoneurones to axotomy. J Physiol. 1974 Aug;240(3):725–739. doi: 10.1113/jphysiol.1974.sp010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Miyata Y., Muñoz-Martinez E. J. Properties of fast and slow alpha motoneurones following motor reinnervation. J Physiol. 1974 Oct;242(1):273–288. doi: 10.1113/jphysiol.1974.sp010706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman A. R. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- Loeb G. E., Duysens J. Activity patterns in individual hindlimb primary and secondary muscle spindle afferents during normal movements in unrestrained cats. J Neurophysiol. 1979 Mar;42(2):420–440. doi: 10.1152/jn.1979.42.2.420. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Munson J. B., Scott J. G. Alterations of synapses on axotomized motoneurones. J Physiol. 1976 Feb;255(1):67–79. doi: 10.1113/jphysiol.1976.sp011270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell L. M., Scott J. G. The effect of peripheral nerve cross-union on connections of single Ia fibers to motoneurons. Exp Brain Res. 1975 Mar 27;22(3):221–234. doi: 10.1007/BF00234765. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Westerman R. A., Ziccone S. P. Discharges of single hindlimb afferents in the freely moving cat. J Neurophysiol. 1976 Sep;39(5):1090–1104. doi: 10.1152/jn.1976.39.5.1090. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Westerman R. A., Ziccone S. P. Ia afferent activity during a variety of voluntary movements in the cat. J Physiol. 1977 Jun;268(2):423–448. doi: 10.1113/jphysiol.1977.sp011864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severin F. V., Orlovskii G. N., Shik M. L. Rabota myshechnykh retseptorov pri upravliaemoi lokomotsii. Biofizika. 1967 May-Jun;12(3):502–511. [PubMed] [Google Scholar]
- Stein R. B., Charles D., Davis L., Jhamandas J., Mannard A., Nichols T. R. Principles underlying new methods for chronic neural recording. Can J Neurol Sci. 1975 Aug;2(3):235–244. doi: 10.1017/s0317167100020333. [DOI] [PubMed] [Google Scholar]
- Stein R. B., Charles D., Gordon T., Hoffer J. A., Jhamandas J. Impedance properties of metal electrodes for chronic recording from mammalian nerves. IEEE Trans Biomed Eng. 1978 Nov;25(6):532–537. doi: 10.1109/TBME.1978.326287. [DOI] [PubMed] [Google Scholar]
- Stein R. B., Nichols T. R., Jhamandas J., Davis L., Charles D. Stable long-term recordings from cat peripheral nerves. Brain Res. 1977 Jun 3;128(1):21–38. doi: 10.1016/0006-8993(77)90233-5. [DOI] [PubMed] [Google Scholar]
- Stein R. B., Oğuztöreli M. N. The radial decline of nerve impulses in a restricted cylindrical extracellular space. Biol Cybern. 1978 Feb 15;28(3):159–165. doi: 10.1007/BF00337137. [DOI] [PubMed] [Google Scholar]
- Sumner B. E. A quantitative analysis of the response of presynaptic boutons to postsynaptic motor neuron axotomy. Exp Neurol. 1975 Mar;46(3):605–615. doi: 10.1016/0014-4886(75)90129-6. [DOI] [PubMed] [Google Scholar]
- Sumner B. E., Sutherland F. I. Quantitative electron microscopy on the injured hypoglossal nucleus in the rat. J Neurocytol. 1973 Sep;2(3):315–328. doi: 10.1007/BF01104033. [DOI] [PubMed] [Google Scholar]
- Sumner B. E., Watson W. E. Retraction and expansion of the dendritic tree of motor neurones of adult rats induced in vivo. Nature. 1971 Sep 24;233(5317):273–275. doi: 10.1038/233273a0. [DOI] [PubMed] [Google Scholar]
- Viala G., Buser P. Activités locomotrices rythmiques stéréotypées chez le lapin sous anesthésie légère. Etude de leurs caractéristiques générales. Exp Brain Res. 1969;8(4):346–363. doi: 10.1007/BF00234381. [DOI] [PubMed] [Google Scholar]
- Walsh J. V., Jr, Burke R. E., Rymer W. Z., Tsairis P. Effect of compensatory hypertrophy studied in individual motor units in medial gastrocnemius muscle of the cat. J Neurophysiol. 1978 Mar;41(2):496–508. doi: 10.1152/jn.1978.41.2.496. [DOI] [PubMed] [Google Scholar]


