Abstract
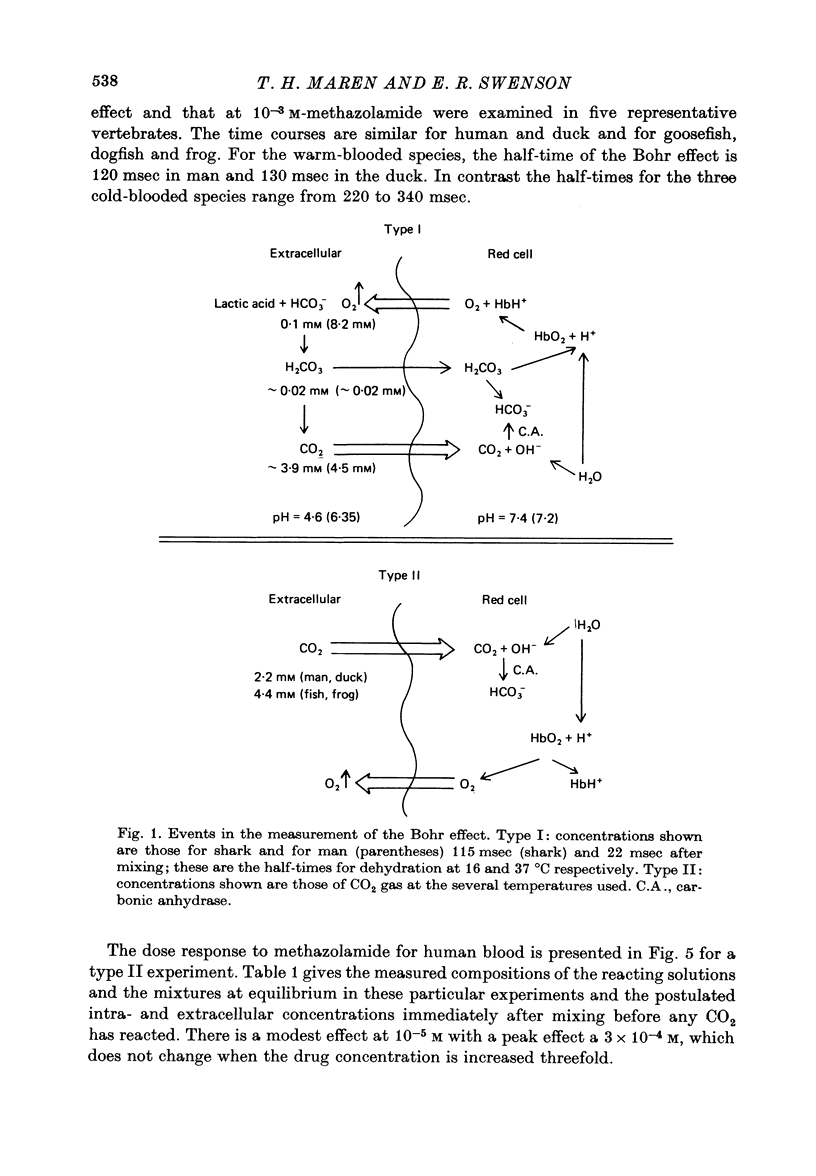
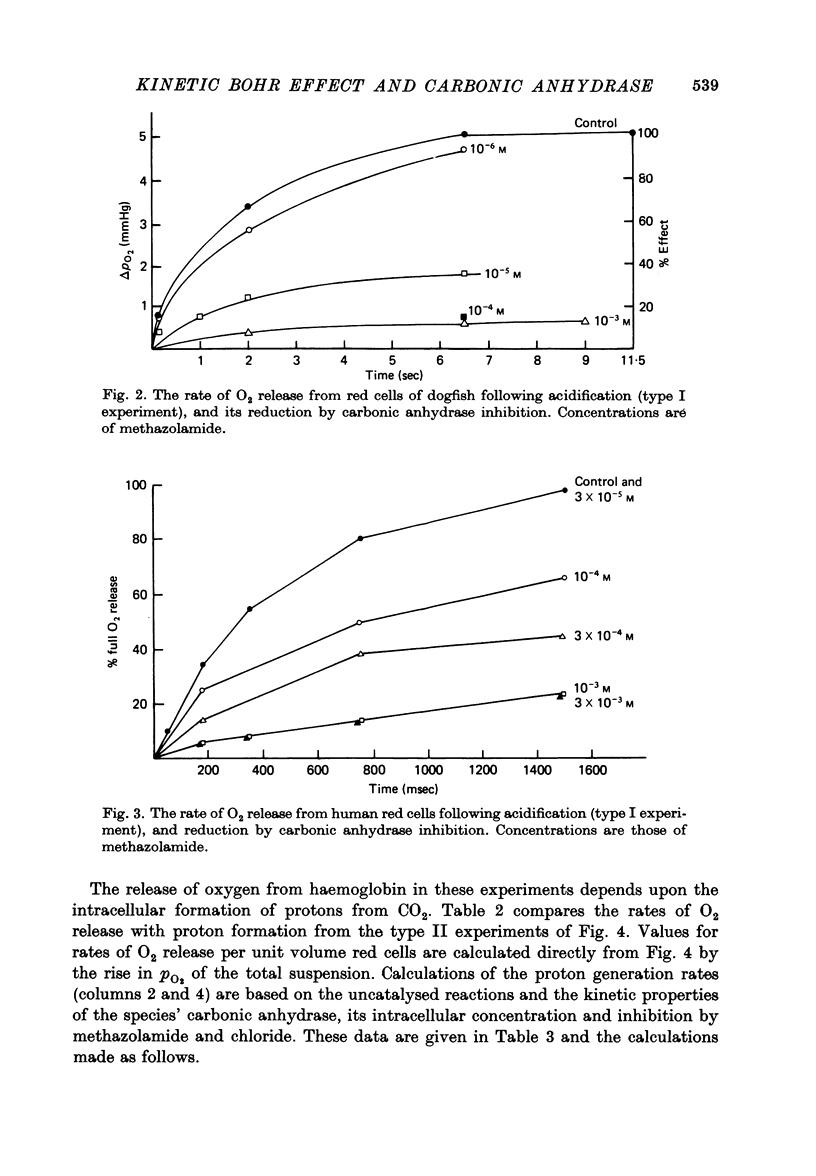
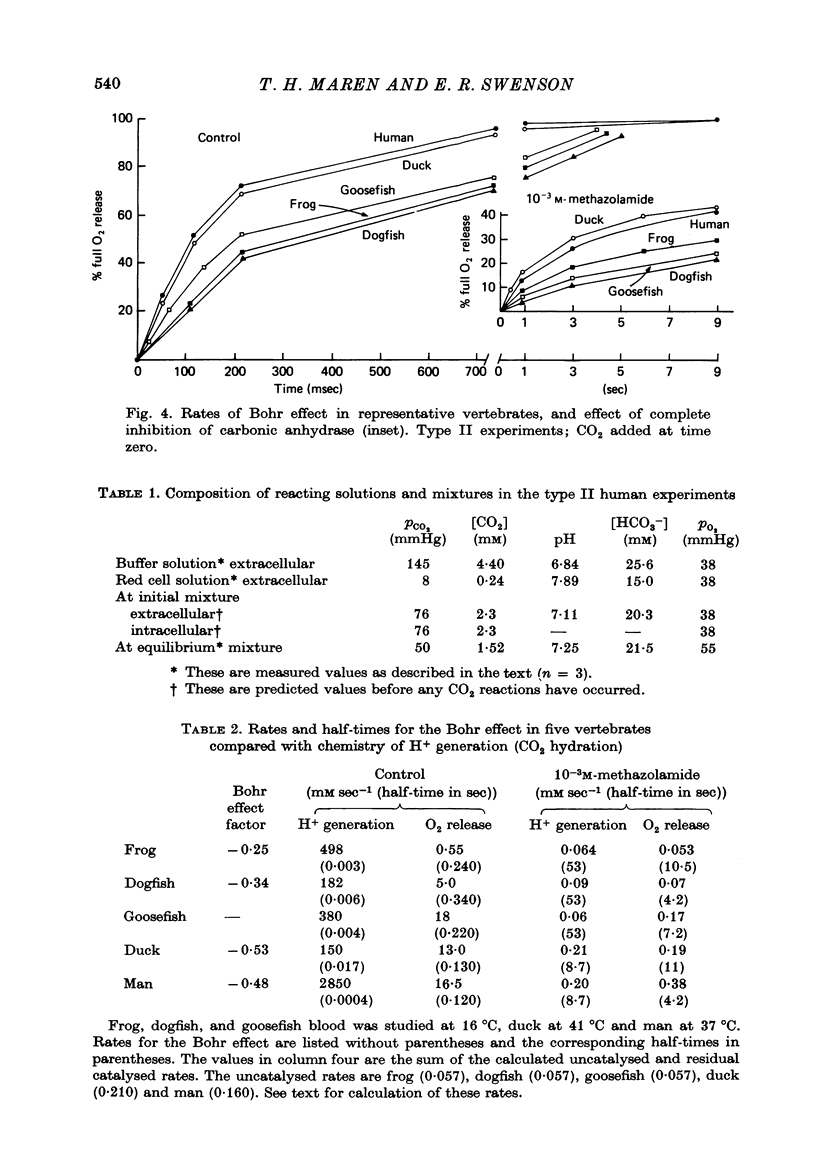
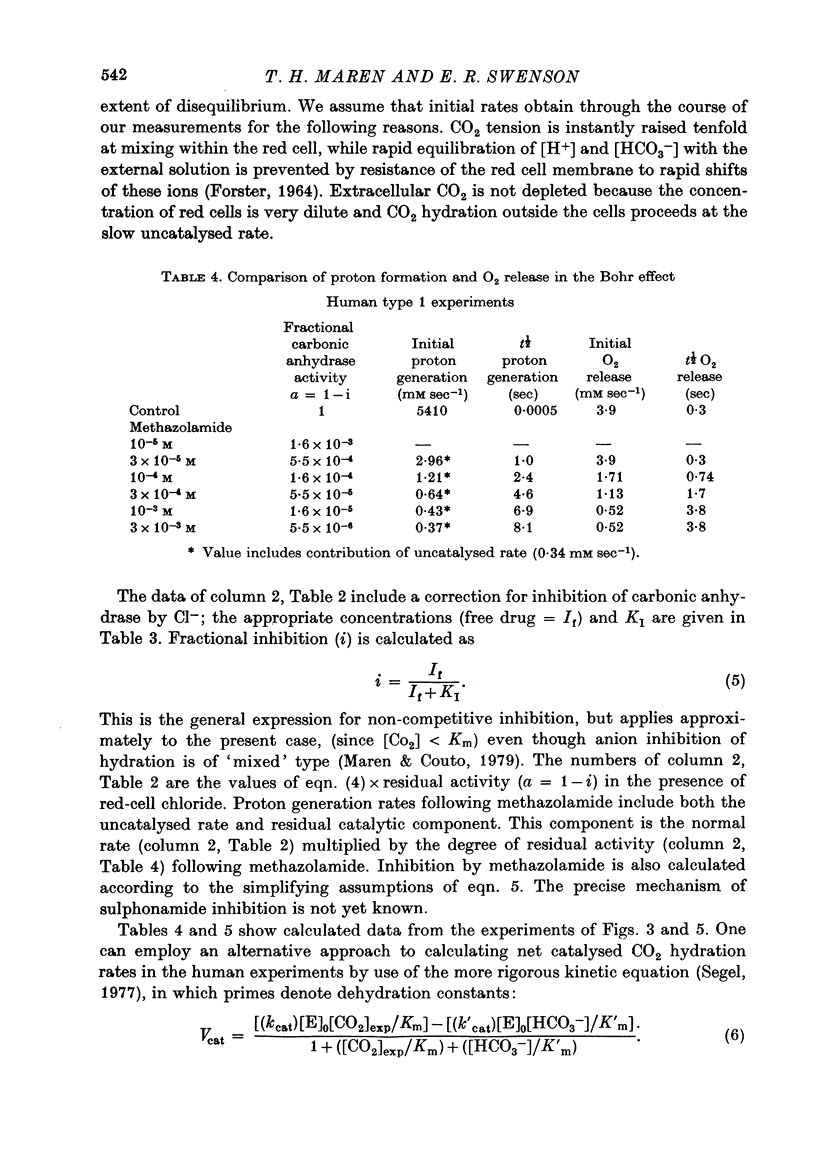
1. The kinetics of the Bohr effect and the role of carbonic anhydrase were studied in a series of representative vertebrates using a continuous flow rapid reaction apparatus. 2. The rates of the Bohr effect in vertebrates are very similar, and differences among classes are manifestations of the ambient temperature. 3. Complete carbonic anhydrase inhibition causes a fifteen to fortyfold reduction in the rate of the Bohr effect, sufficient to abolish its occurrence within capillary transit. 4. There is a twenty-three-fold (duck) to 360-fold (man) excess of carbonic anhydrase activity in vertebrate red cells for the normal generation of the Bohr effect. 5. When carbonic anhydrase is inhibited and CO2 hydration becomes rate limiting, the stoichiometry of the Bohr effect (delta log pO2/delta pH) is revealed in the ratio of the rates of proton formation in red cells to O2 release from haemoglobin.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein R. S., Schraer R. Purification and properties of an avian carbonic anhydrase from the erythrocytes of Gallus domesticus. J Biol Chem. 1972 Feb 25;247(4):1306–1322. [PubMed] [Google Scholar]
- Bundy H. F., Cheng B. Amphibian carbonic anhydrase: purification and partial characterization of the enzyme from erythrocytes of Rana catesbeiana. Comp Biochem Physiol B. 1976;55(2):265–271. doi: 10.1016/0305-0491(76)90243-1. [DOI] [PubMed] [Google Scholar]
- CRAW M. R., CONSTANTINE H. P., MORELLO J. A., FORSTER R. E. Rate of the Bohr shift in human red cell suspensions. J Appl Physiol. 1963 Mar;18:317–324. doi: 10.1152/jappl.1963.18.2.317. [DOI] [PubMed] [Google Scholar]
- Chow E. I., Crandall E. D., Forster R. E. Kinetics of bicarbonate-chloride exchange across the human red blood cell membrane. J Gen Physiol. 1976 Dec;68(6):633–652. doi: 10.1085/jgp.68.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R. E., Steen J. B. Rate limiting processes in the Bohr shift in human red cells. J Physiol. 1968 Jun;196(3):541–562. doi: 10.1113/jphysiol.1968.sp008522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILPERT P., FLEISCHMANN R. G., KEMPE D., BARTELS H. THE BOHR EFFECT RELATED TO BLOOD AND ERYTHROCYTE PH. Am J Physiol. 1963 Aug;205:337–340. doi: 10.1152/ajplegacy.1963.205.2.337. [DOI] [PubMed] [Google Scholar]
- Hlastala M. P., Woodson R. D. Saturation dependency of the Bohr effect: interactions among H-+, CO2, and DPG. J Appl Physiol. 1975 Jun;38(6):1126–1131. doi: 10.1152/jappl.1975.38.6.1126. [DOI] [PubMed] [Google Scholar]
- Laux B. E., Raichle M. E. The effect of acetazolamide on cerebral blood flow and oxygen utilization in the rhesus monkey. J Clin Invest. 1978 Sep;62(3):585–592. doi: 10.1172/JCI109164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAREN T. H. A simplified micromethod for the determination of carbonic anhydrase and its inhibitors. J Pharmacol Exp Ther. 1960 Sep;130:26–29. [PubMed] [Google Scholar]
- Magid E. Determination of erythrocyte carbonic anhydrase B and C: an aid in the diagnosis of thyroid disorders? Scand J Clin Lab Invest. 1970 Nov;26(3):257–262. doi: 10.3109/00365517009046231. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Bicarbonate formation in cerebrospinal fluid: role in sodium transport and pH regulation. Am J Physiol. 1972 Apr;222(4):885–899. doi: 10.1152/ajplegacy.1972.222.4.885. [DOI] [PubMed] [Google Scholar]
- Maren T. H., Couto E. O. The nature of anion inhibition of human red cell carbonic anhydrases. Arch Biochem Biophys. 1979 Sep;196(2):501–510. doi: 10.1016/0003-9861(79)90302-3. [DOI] [PubMed] [Google Scholar]
- Maren T. H., Haywood J. R., Chapman S. K., Zimmerman T. J. The pharmacology of methazolamide in relation to the treatment of glaucoma. Invest Ophthalmol Vis Sci. 1977 Aug;16(8):730–742. [PubMed] [Google Scholar]
- Maren T. H., Rayburn C. S., Liddell N. E. Inhibition by anions of human red cell carbonic anhydrase B: physiological and biochemical implications. Science. 1976 Feb 6;191(4226):469–472. doi: 10.1126/science.813299. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Use of inhibitors in physiological studies of carbonic anhydrase. Am J Physiol. 1977 Apr;232(4):F291–F297. doi: 10.1152/ajprenal.1977.232.4.F291. [DOI] [PubMed] [Google Scholar]
- Riggs A. The Nature and Significance of the Bohr Effect in Mammalian Hemoglobins. J Gen Physiol. 1960 Mar 1;43(4):737–752. doi: 10.1085/jgp.43.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughton F. J. Some recent work on the interactions of oxygen, carbon dioxide and haemoglobin. Biochem J. 1970 May;117(5):801–812. doi: 10.1042/bj1170801b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. N., Tu C., Wynns G. C. Proton transfer between hemoglobin and the carbonic anhydrase active site. J Biol Chem. 1978 Apr 25;253(8):2563–2567. [PubMed] [Google Scholar]
- Swenson E. R., Maren T. H. A quantitative analysis of CO2 transport at rest and during maximal exercise. Respir Physiol. 1978 Nov;35(2):129–159. doi: 10.1016/0034-5687(78)90018-x. [DOI] [PubMed] [Google Scholar]
- WYMAN J., Jr LINKED FUNCTIONS AND RECIPROCAL EFFECTS IN HEMOGLOBIN: A SECOND LOOK. Adv Protein Chem. 1964;19:223–286. doi: 10.1016/s0065-3233(08)60190-4. [DOI] [PubMed] [Google Scholar]


