Abstract
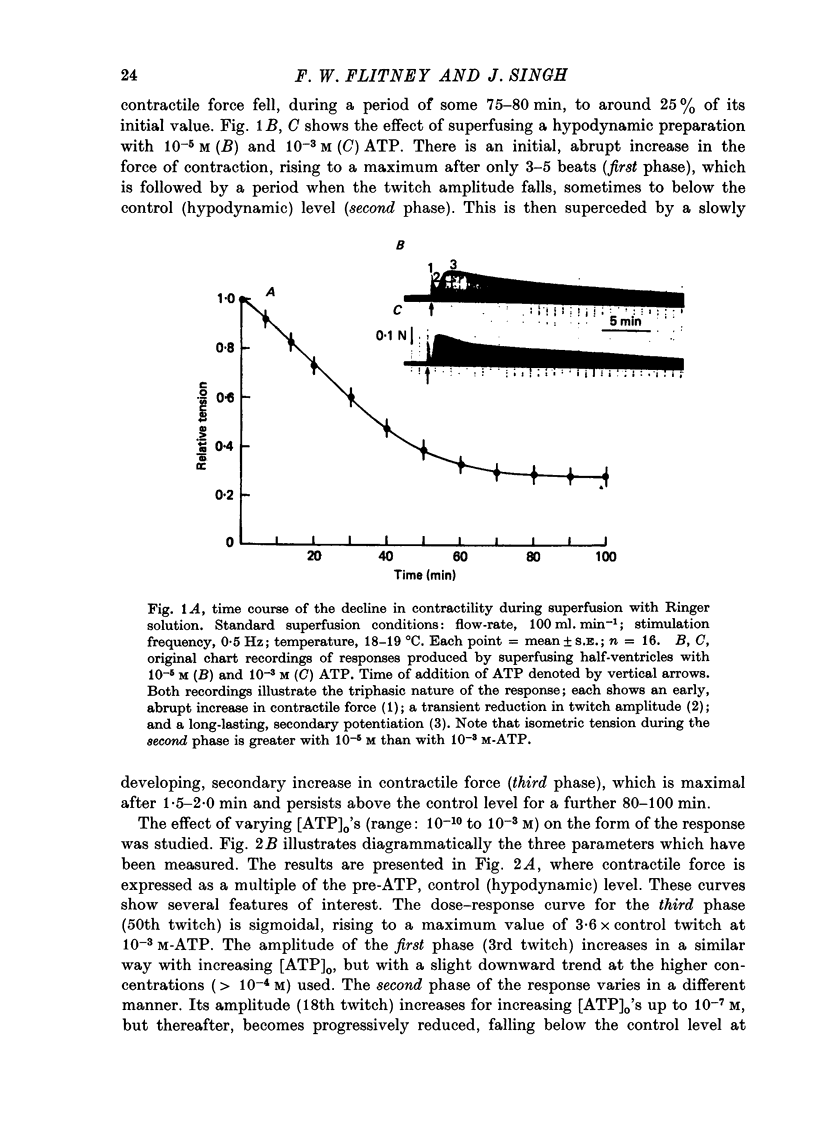
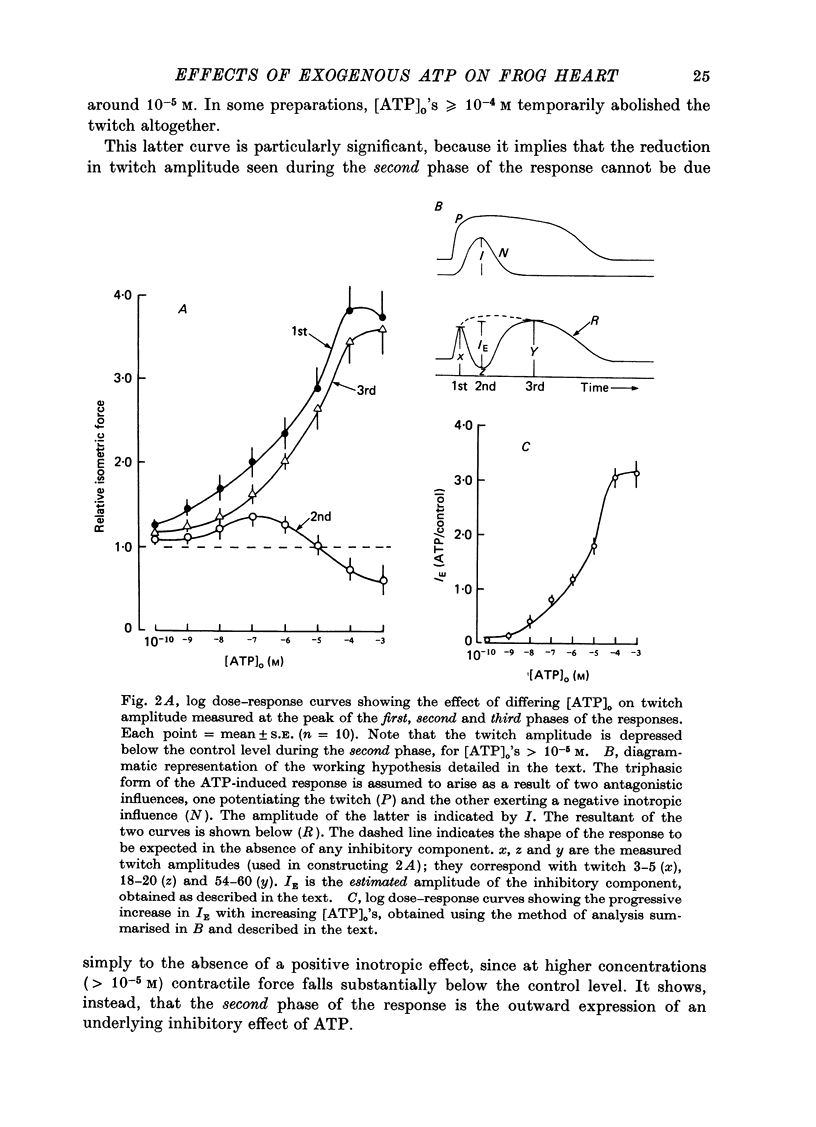
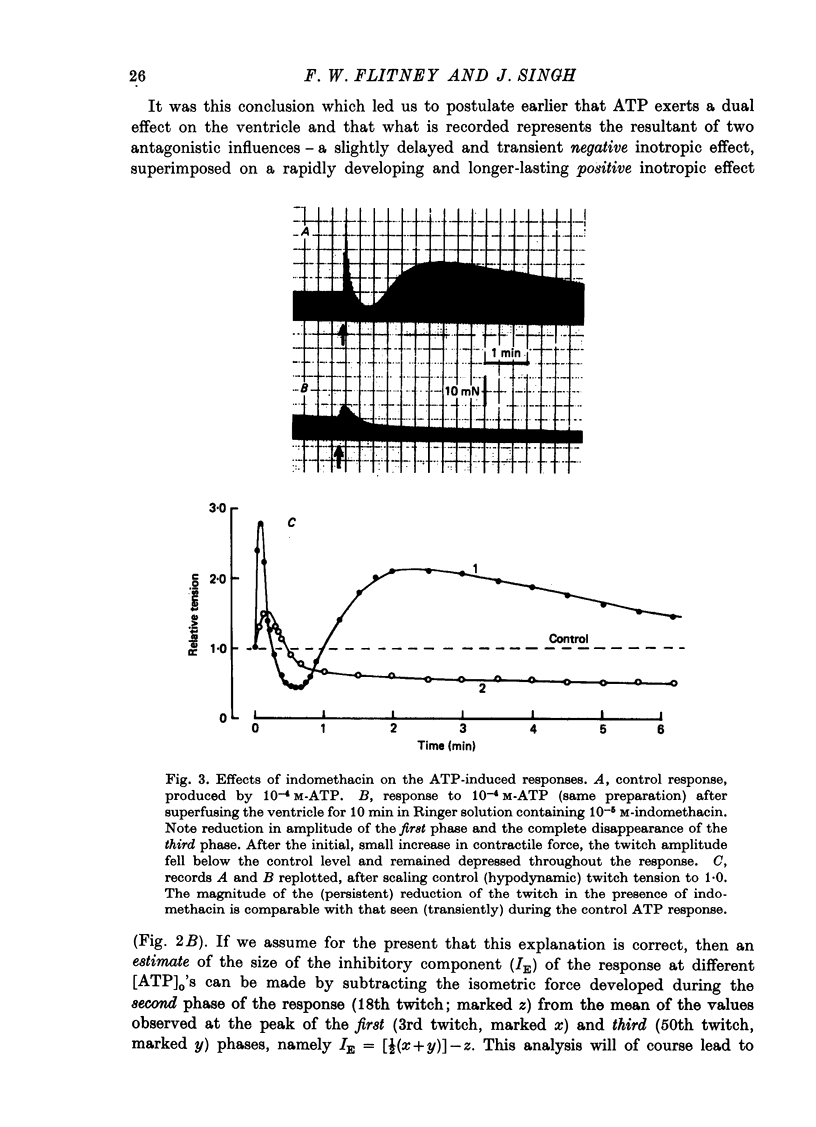
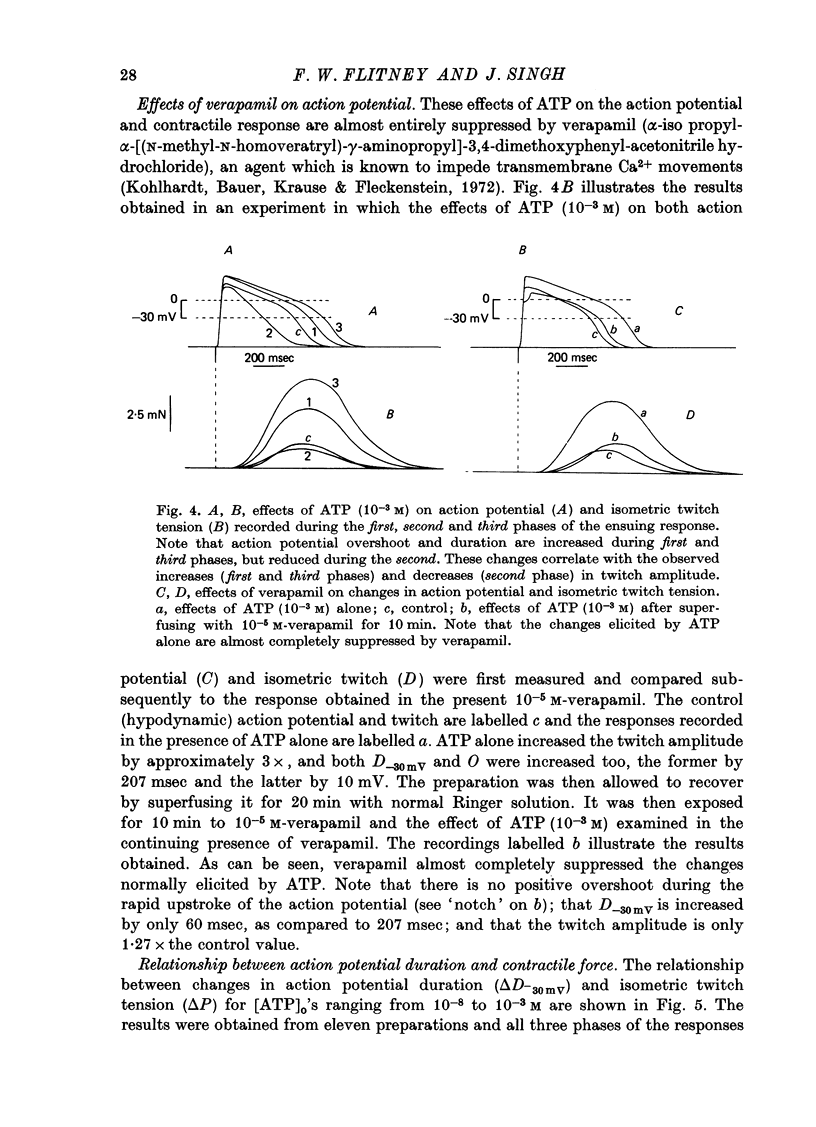
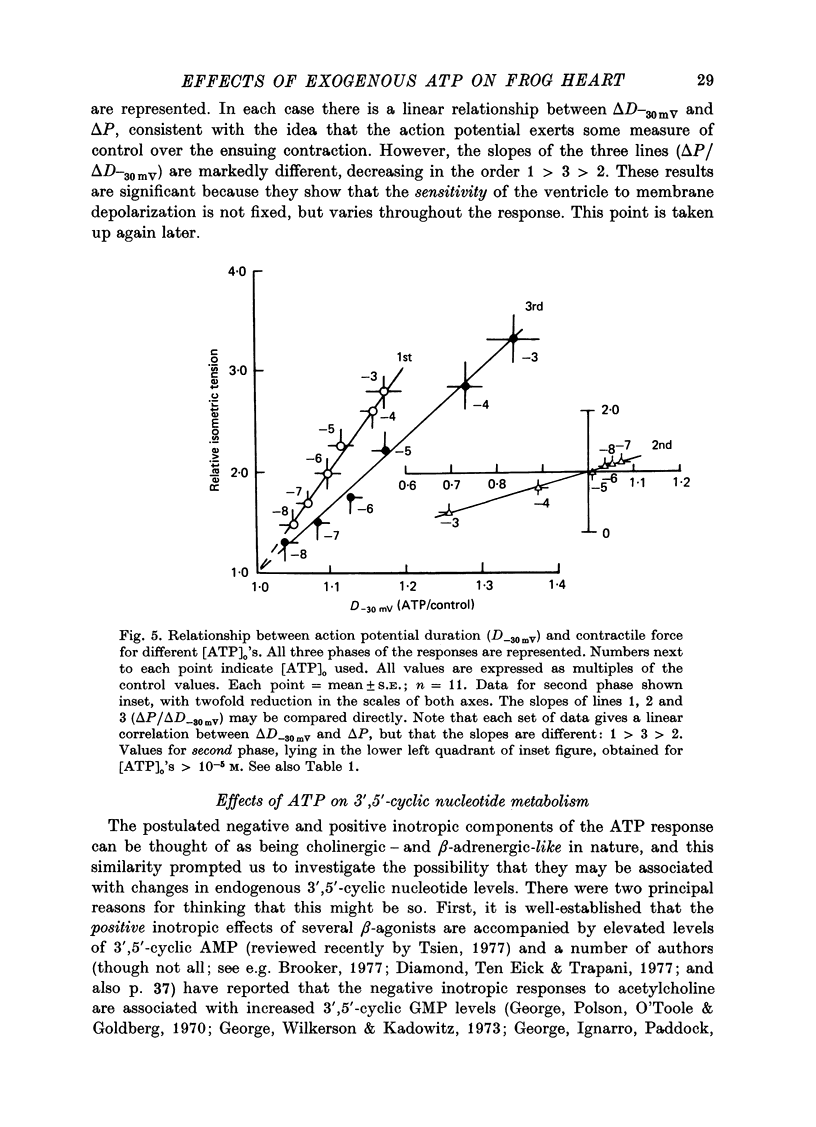
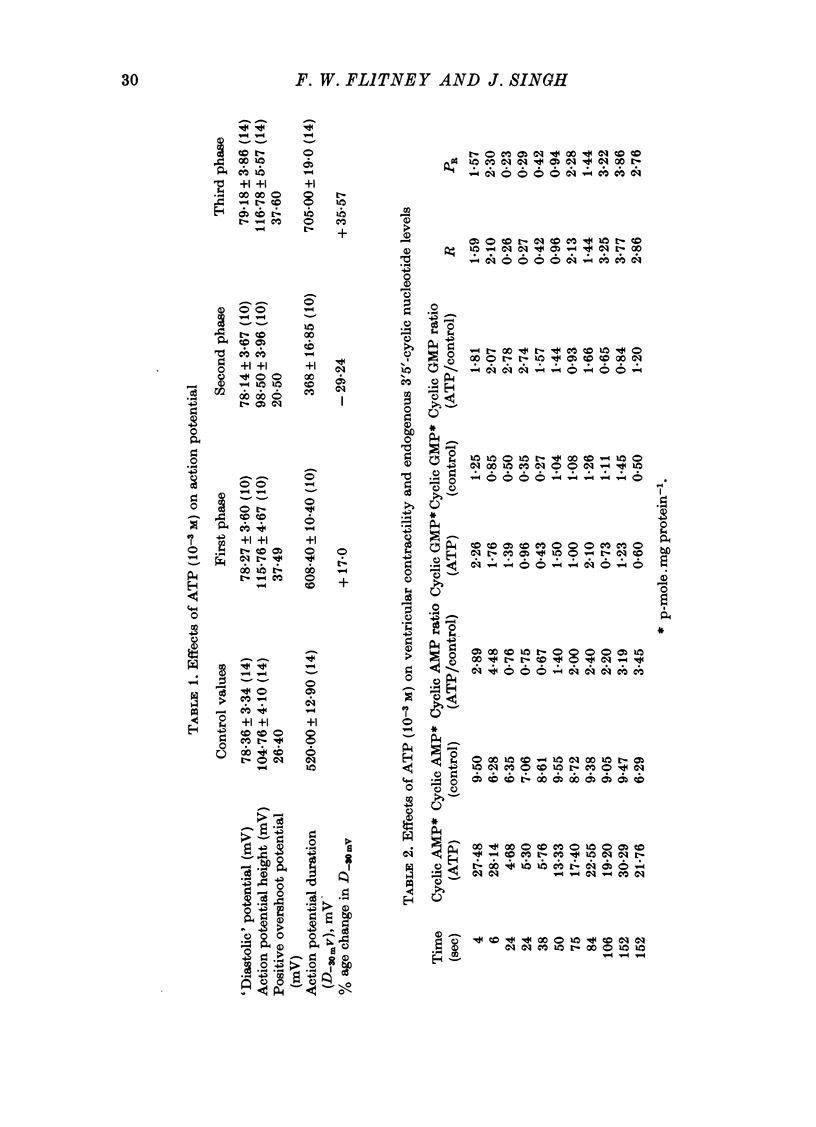
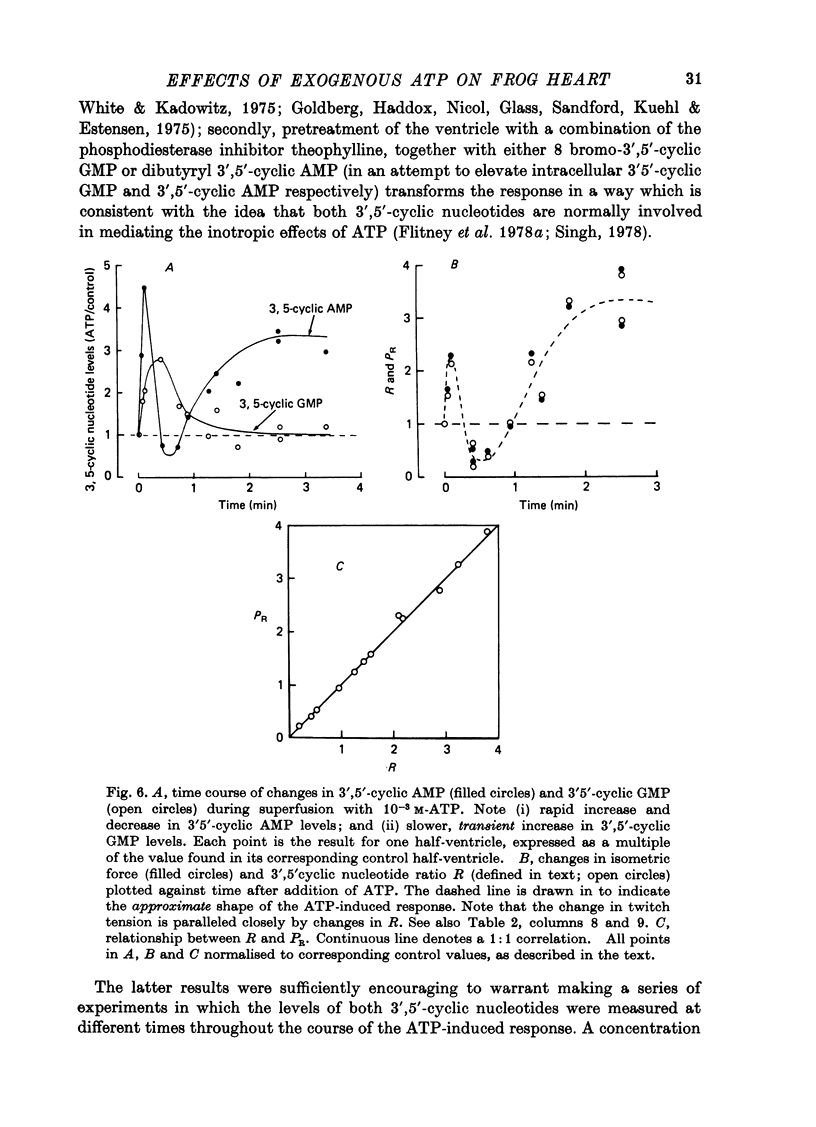
1. A study has been made of a well documented but poorly understood response of the isolated frog ventricle to treatment with exogenous adenosine 5' triphosphate (ATP). Measurements of membrane potential, isometric twitch tension and levels of endogenous 3',5'-cyclic nucleotides have been made at various times during the ATP-induced response. 2. ATP elicits a characteristic triphasic response, which comprises an initial, abrupt increase in contractility, rising to a maximum within a few beats (first phase); followed by a period when the twitch amplitude falls, sometimes to below the control level (second phase); and superceded by a more slowly developing and longer-lasting increase in contractile force (third phase). The response is unaffected by atropine, propranolol or phentolamine. However, the prostaglandin synthetase inhibitor indomethacin depresses the first phase and entirely suppresses the third phase. 3. The inotropic effects of ATP are accompanied by changes in the shape of the action potential. These effects are dose-related. The duration of the action potential (D-30mV) and its positive overshoot (O) are increased during all phases of the response, for [ATP]o's up to 10(-5) M. However, at higher [ATP]o's, D-30mV and O ar both reduced during the second phase (but not the first or third phase), when isometric twitch tension is also depressed. The relationship between action potential duration and twitch tension (P) for different [ATP]o's is linear for all three phases of the response, but the slopes of the curves (delta P/delta D) are markedly different, indicating that the sensitivity of the contractile system to membrane depolarization is not constant, but varies continuously throughout the response. 4. ATP has a potent stimulatory effect on the metabolism of endogenous 3',5'-cyclic nucleotides. The time courses of the changes in adenosine 3','5-cyclic monophosphate (3',5'-cyclic AMP) and guanosine 3',5'-cyclic monophosphate (3',5'-cyclic GMP) are complex, but the accompanying change in isometric twitch tension is paralleled closely by corresponding changes in the ratio 3',5'cyclic AMP:3',5'-cyclic GMP. 5. It is concluded that ATP exerts a dual effect on the ventricle and that the contractile response is regulated by changes in the metabolism of 3',5'-cyclic nucleotides. The effects of indomethacin indicate a possible involvement of prostaglandins in mediating the ATP response. It is suggested that the initial effect of ATP on the ventricle is to increase the permeability of the fibres to Ca2+. 6. The relationship between 3',5' cyclic nucleotide levels and ventricular contractility is discussed. It is postulated that the antagonistic effects of 3',5'-cyclic AMP and 3',5'-cyclic GMP are expressed at the level of certain phosphoproteins which regulate both the availability of Ca2+ and the sensitivity of the contractile proteins to Ca2+.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACIERNO L., BURNO F., BURSTEIN F., DI PLAMA J. R. Actions of adenosine triphosphate on the isolated cat atrium and their antagonism by acetylcholine. J Pharmacol Exp Ther. 1952 Mar;104(3):264–268. [PubMed] [Google Scholar]
- ANGELAKOS E. T., GLASSMAN P. M. Cardiovascular action of adenosine and other nucleosides. Proc Soc Exp Biol Med. 1961 Apr;106:762–763. doi: 10.3181/00379727-106-26467. [DOI] [PubMed] [Google Scholar]
- Arch J. R., Newsholme E. A. The control of the metabolism and the hormonal role of adenosine. Essays Biochem. 1978;14:82–123. [PubMed] [Google Scholar]
- Bielschowsky M., Green H. N., Stoner H. B. The effect of magnesium and calcium on the physiological properties of certain purine derivatives. J Physiol. 1946 Jan 15;104(3):239–252. doi: 10.1113/jphysiol.1946.sp004118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd I. A., Forrester T. The release of adenosine triphosphate from frog skeletal muscle in vitro. J Physiol. 1968 Nov;199(1):115–135. doi: 10.1113/jphysiol.1968.sp008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker G. Dissociation of cyclic GMP from the negative inotropic action of carbachol in guinea pig atria. J Cyclic Nucleotide Res. 1977 Dec;3(6):407–413. [PubMed] [Google Scholar]
- Brown C., Burnstock G., Cocks T. Effects of adenosine 5'-triphosphate (ATP) and beta-gamma-methylene ATP on the rat urinary bladder. Br J Pharmacol. 1979 Jan;65(1):97–102. doi: 10.1111/j.1476-5381.1979.tb17337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudry I. H., Gould M. K. Evidence for the uptake of ATP by rat soleus muscle in vitro. Biochim Biophys Acta. 1970;196(2):320–326. doi: 10.1016/0005-2736(70)90019-2. [DOI] [PubMed] [Google Scholar]
- Cole H. A., Perry S. V. The phosphorylation of troponin I from cardiac muscle. Biochem J. 1975 Sep;149(3):525–533. doi: 10.1042/bj1490525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J., Ten Eick R. E., Trapani A. J. Are increases in cyclic GMP levels responsible for the negative inotropic effects of acetylcholine in the heart? Biochem Biophys Res Commun. 1977 Dec 7;79(3):912–918. doi: 10.1016/0006-291x(77)91197-4. [DOI] [PubMed] [Google Scholar]
- Drury A. N., Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929 Nov 25;68(3):213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England P. J. Correlation between contraction and phosphorylation of the inhibitory subunit of troponin in perfused rat heart. FEBS Lett. 1975 Jan 15;50(1):57–60. doi: 10.1016/0014-5793(75)81040-4. [DOI] [PubMed] [Google Scholar]
- England P. J. Studies on the phosphorylation of the inhibitory subunit of troponin during modification of contraction in perfused rat heart. Biochem J. 1976 Nov 15;160(2):295–304. doi: 10.1042/bj1600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitney F. W., Lamb J. F., Singh J. Effects of ATP on the hypodynamic frog ventricle [proceedings]. J Physiol. 1977 Dec;273(2):50P–52P. [PubMed] [Google Scholar]
- Flitney F. W., Lamb J. F., Singh J. Effects of exogenous ATP on contractile force and intracellular cyclic nucleotide levels in the hypodynamic frog ventricle [proceedings]. J Physiol. 1978 Apr;277:69P–70P. [PubMed] [Google Scholar]
- Flitney F. W., Lamb J. F., Singh J. Intracellular cyclic nucleotides and contractility of the hypodynamic frog ventricle [proceedings]. J Physiol. 1978 Mar;276:38P–39P. [PubMed] [Google Scholar]
- Flitney F. W., Singh J. Exogenous uridine 5'-triphosphate enhances contractility and stimulates 3',5'-cyclic nucleotide metabolism in the isolated frog ventricle [proceedings]. J Physiol. 1979 Jun;291:52P–53P. [PubMed] [Google Scholar]
- Flitney F. W., Singh J. Release of prostaglandins from the isolated frog ventricle and associated changes in endogenous cyclic nucleotide levels. J Physiol. 1980 Jul;304:1–20. doi: 10.1113/jphysiol.1980.sp013306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester T., Hamilton I. J. Proceedings: Functional hyperaemia in soleus muscle of the cat. J Physiol. 1975 Jul;249(1):20P–21P. [PMC free article] [PubMed] [Google Scholar]
- Forrester T., Williams C. A. Release of adenosine triphosphate from isolated adult heart cells in response to hypoxia. J Physiol. 1977 Jun;268(2):371–390. doi: 10.1113/jphysiol.1977.sp011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George W. J., Ignarro L. J., Paddock R. J., White L., Kadowitz P. J. Oppositional effects of acetylcholine and isoproterenol on isometric tension and cyclic nucleotide concentrations in rabbit atria. J Cyclic Nucleotide Res. 1975;1(5):339–347. [PubMed] [Google Scholar]
- George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. Elevation of guanosine 3',5'-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci U S A. 1970 Jun;66(2):398–403. doi: 10.1073/pnas.66.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George W. J., Wilkerson R. D., Kadowitz P. J. Influence of acetylcholine on contractile force and cyclic nucleotide levels in the isolated perfused rat heart. J Pharmacol Exp Ther. 1973 Jan;184(1):228–235. [PubMed] [Google Scholar]
- Giles W., Noble S. J. Changes in membrane currents in bullfrog atrium produced by acetylcholine. J Physiol. 1976 Sep;261(1):103–123. doi: 10.1113/jphysiol.1976.sp011550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. H. The biological significance of the linkages in adenosine triphosphoric acid. J Physiol. 1934 Feb 28;80(4):345–359. doi: 10.1113/jphysiol.1934.sp003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M. Membrane adenosine triphosphatase and cation transport. Br Med Bull. 1968 May;24(2):165–169. doi: 10.1093/oxfordjournals.bmb.a070620. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K., Nicol S. E., Glass D. B., Sanford C. H., Kuehl F. A., Jr, Estensen R. Biologic regulation through opposing influences of cyclic GMP and cyclic AMP: the Yin Yang hypothesis. Adv Cyclic Nucleotide Res. 1975;5:307–330. [PubMed] [Google Scholar]
- Goto M., Yatani A., Tsuda Y. An analysis of the action of ATP and related compounds on membrane current and tension components in bullfrog atrial muscle. Jpn J Physiol. 1977;27(1):81–94. doi: 10.2170/jjphysiol.27.81. [DOI] [PubMed] [Google Scholar]
- HOFFMANN P. C., OKITA G. T. PENETRATION OF ATP INTO THE MYOCARDIUM. Proc Soc Exp Biol Med. 1965 Jun;119:573–576. doi: 10.3181/00379727-119-30242. [DOI] [PubMed] [Google Scholar]
- HOLLANDER P. B., WEBB J. L. Effects of adenine nucleotides on the contractility and membrane potentials of rat atrium. Circ Res. 1957 Jul;5(4):349–353. doi: 10.1161/01.res.5.4.349. [DOI] [PubMed] [Google Scholar]
- Hattori E., Miyazaki T., Nakamura M. Incorporation of adenosine and adenosine triphosphate into rat myocardium. Jpn Heart J. 1969 Jan;10(1):47–52. doi: 10.1536/ihj.10.47. [DOI] [PubMed] [Google Scholar]
- Ikemoto Y., Goto M. Effects of ACh on slow inward current and tension components of the bullfrog atrium. J Mol Cell Cardiol. 1977 Apr;9(4):313–326. doi: 10.1016/s0022-2828(77)80037-0. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Tada M., Kirchberger M. A. Control of calcium transport in the myocardium by the cyclic AMP-Protein kinase system. Adv Cyclic Nucleotide Res. 1975;5:453–472. [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Differentiation of the transmembrane Na and Ca channels in mammalian cardiac fibres by the use of specific inhibitors. Pflugers Arch. 1972;335(4):309–322. doi: 10.1007/BF00586221. [DOI] [PubMed] [Google Scholar]
- Kuo J. F. Guanosine 3':5'-monophosphate-dependent protein kinases in mammalian tissues. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4037–4041. doi: 10.1073/pnas.71.10.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LICHTNECKERT I., STRAUB F. B. The action of adenosinetriphosphate on the isolated frog heart. Hung Acta Physiol. 1949;2(1-4):50–57. [PubMed] [Google Scholar]
- MARSHALL J. M., ANDRUS E. C. Comparison of effects of various phosphate compounds and aluminum silicate on isolated frog heart. Proc Soc Exp Biol Med. 1953 Feb;82(2):228–231. doi: 10.3181/00379727-82-20074. [DOI] [PubMed] [Google Scholar]
- Meinertz T., Nawrath H., Scholz H. Influence of cyclization and acyl substitution on the inotropic effects of adenine nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 1973;278(2):165–178. doi: 10.1007/BF00500648. [DOI] [PubMed] [Google Scholar]
- Morad M., Orkand R. K. Excitation-concentration coupling in frog ventricle: evidence from voltage clamp studies. J Physiol. 1971 Dec;219(1):167–189. doi: 10.1113/jphysiol.1971.sp009656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedergerke R., Orkand R. K. The dual effect of calcium on the action potential of the frog's heart. J Physiol. 1966 May;184(2):291–311. doi: 10.1113/jphysiol.1966.sp007916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddle B. M., Burnstock G. Release of ATP from perfused heart during coronary vasodilatation. Blood Vessels. 1974;11(3):110–119. doi: 10.1159/000158005. [DOI] [PubMed] [Google Scholar]
- RAND M., STAFFORD A., THORP R. H. The potentiation of the action of adenosine on the guinea pig heart by ouabain. J Pharmacol Exp Ther. 1955 May;114(1):119–125. [PubMed] [Google Scholar]
- Ray K. P., England P. J. Phosphorylation of the inhibitory subunit of troponin and its effect on the calcium dependence of cardiac myofibril adenosine triphosphatase. FEBS Lett. 1976 Nov;70(1):11–16. doi: 10.1016/0014-5793(76)80716-8. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977 Jan;264(1):49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHENBERG S. Positive inotropic response of amphibian hearts to adenosine nucleotides. Acta Physiol Lat Am. 1956;6(4):137–146. [PubMed] [Google Scholar]
- SYDOW V., AHLQUIST R. P. Cardiovascular actions of some simple nucleic acid derivatives. J Am Pharm Assoc Am Pharm Assoc. 1954 Mar;43(3):166–170. doi: 10.1002/jps.3030430313. [DOI] [PubMed] [Google Scholar]
- Singh J., Flitney F. W., Lamb J. F. Effects of isoprenaline on contractile force and intracellular cyclic 3',5'-nucleotide levels in the hypodynamic frog ventricle. FEBS Lett. 1978 Jul 15;91(2):269–272. doi: 10.1016/0014-5793(78)81189-2. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Moir A. J., Perry S. V. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature. 1976 Aug 12;262(5569):615–617. doi: 10.1038/262615a0. [DOI] [PubMed] [Google Scholar]
- Tada M., Yamamoto T., Tonomura Y. Molecular mechanism of active calcium transport by sarcoplasmic reticulum. Physiol Rev. 1978 Jan;58(1):1–79. doi: 10.1152/physrev.1978.58.1.1. [DOI] [PubMed] [Google Scholar]
- VERSPRILLE A. DIFFERENCES BETWEEN THE CHRONOTROPIC EFFECT OF ATP ON THE ISOLATED FROG HEART AND THE ISOLATED FROG VENTRICLE. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965 Feb 5;283:82–88. doi: 10.1007/BF00363303. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Vassort G., Rougier O., Garnier D., Sauviat M. P., Coraboeuf E., Gargouïl Y. M. Effects of adrenaline on membrane inward currents during the cardiac action potential. Pflugers Arch. 1969;309(1):70–81. doi: 10.1007/BF00592283. [DOI] [PubMed] [Google Scholar]
- WAYNE E. J., GOODWIN J. F., STONER H. B. The effect of adenosine triphosphate on the electrocardiogram of man and animals. Br Heart J. 1949 Jan;11(1):55–67. doi: 10.1136/hrt.11.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberger A., Will H. Protein kinase-catalyzed membrane phosphorylation and its possible relationship to the role of calcium in the adrenergic regulation of cardiac contraction. Life Sci. 1978 Apr 3;22(13-15):1159–1178. doi: 10.1016/0024-3205(78)90085-1. [DOI] [PubMed] [Google Scholar]
- Yatani A., Goto M., Tsuda Y. Nature of catecholamine-like actions of ATP and other energy rich nucleotides on the bullfrog atrial muscle. Jpn J Physiol. 1978;28(1):47–61. doi: 10.2170/jjphysiol.28.47. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]


